in which PB is the amount of protein bound (g/kg DM) and PPP is plant protein-precipitable phenolics (% DM). The critical percentage of dietary PPP that occurred at the intersection of the linear response and the plateau line was 6.4%. The maximum amount of protein bound by a mixture of PPP was estimated as 37.24 g protein/kg DM. Condensed tannins account for 81% of the variation that occurred in the observed values of protein bound.
It is well established that CT inclusion decreases the rate and extent of ruminal CP degradation (Waghorn, 2008; Patra and Saxena, 2011). Lotus corniculatus is a frequently studied CT-containing forage and is often evaluated as a replacement for Medicago sativa. Lotus corniculatus (0.972.77% CT) linearly decreased the immediately soluble CP fraction and rate of degradation in situ, thus decreasing forage RDP proportion compared with Medicago sativa (0% CT) (Williams et al., 2010; Coblentz and Grabber, 2013). Regressing protein degradation extent on forage CT concentration indicated that each unit of CT protected an average of 0.61 units of CP from ruminal degradation (Coblentz and Grabber, 2013).
Other effective CT-containing forages include Lespedeza stuevei Nutt. and A. angustissima var. hirta, which were identified as plants contributing exceptionally high protection from in vitro ruminal soybean meal protein degradation when mixed in a 1:1 ratio (Johnson et al., 2015). In the same study, the extent of CP degradation at 48 h was inversely related to the concentration of protein-precipitable polyphenols in six warm-season perennial legumes (Johnson et al., 2015).
Similar results are observed when CT extracts are supplemented. Vitis vinifera (grape) seed extract (GSE) complexed with Lupinus angustifolius var. Tanjil seed CP (at ratios of 96 and 180 mg GSE/g CP) decreased the immediately soluble fraction and CP degradation rate in Merino rams, resulting in an increased proportion of RUP with increased CT concentration (Bruno-Soares et al., 2011). However, the effective reduction of CP degradation was not proportional to the two-fold increase in CT (Bruno-Soares et al., 2011). Quebracho tannin extract (QTE) is a commercially available CT source from Schinopsis spp., consistently reported to decrease ruminal CP degradation at multiple dietary fiber (Dschaak et al., 2011) and CP levels (Aguerre et al., 2016). Responses appear to be dose- related as increasing inclusion from 1 to 6% of DM linearly decrease ruminal CP degradation (Ahnert et al., 2015).
Decreased protein degradation in the rumen due to CT supplementation subsequently decreases NH3 concentrations, providing further value to the animal. Rumen available N in excess of microbial growth requirements is absorbed as NH3, metabolized to urea in the liver, and either recycled or excreted in urine. High protein degradation rates increase urinary N excretion, which can negatively impact the environment (Powell et al., 2010), decrease N utilization efficiency, and impart a metabolic burden associated with increased urea synthesis and excretion that hinders animal performance (Van Duinkerken et al., 2005; Kohn et al., 2005) and fertility (Westwood et al., 2000; Tshuma et al., 2014).
Increasing RUP through CT inclusion consistently decreases ruminal NH3-N concentration in vitro and in vivo across multiple species, unless feed protein levels are much greater than the requirements of the animal. A dose response is generally observed and is related to the protein precipitation ability of the CT (Johnson et al., 2015). In a continuous culture model, replacing either half or all of Medicago sativa hay in a dairy ration with L. corniculatus hay (0.32 and 0.97% total dietary CT) decreased NH3-N concentration and flow by at least 15 and 25%, respectively (Williams et al., 2010). Higher CT concentrations in vivo decreased rumen NH3 concentration up to 24% when L. corniculatus silage (7.3-9.5% dietary total CT) replaced M. sativa silage (0% dietary total CT) in a dairy cattle ration (Hymes-Fecht et al., 2013). A few studies however, have reported no effect of CT on ruminal NH3-N concentrations (Williams et al., 2011; Dickhoefer et al., 2016). These results may be explained by CT source, lower water intake [i.e. lower NH3 dilution as in Dickhoefer et al. (2016)] or because energy availability was limiting for microbial growth. Christensen et al. (2015) also reported no differences in rumen NH3-N concentration of lactating dairy cows after replacing 50 or 100% of M. sativa hay with L. corniculatus (0.51% CT). The lack of response appears to be due to high variation (1.244 mg/100 mL) given that NH3-N concentrations were greater in the M. sativa diet (8.33 mg/100 mL) versus the mixed (6.05 mg/100 mL) and L. corniculatus-based (6.70 mg/100 mL) diets (Christensen et al., 2015). However, microbial protein production was improved in a birdsfoot trefoil diet suggesting a more efficient use of dietary N when a CT-containing forage was offered in replacement for alfalfa. As rumen NH3 is reduced, CT shift N excretion from the urine to feces (Deaville et al., 2010; Williams et al., 2011; Hymes-Fecht et al., 2013; Ahnert et al., 2015; Orlandi et al., 2015; Aguerre et al., 2016), which may substantially improve environmental sustainability of ruminant production through reduced nitrous oxide emissions from manure (Powell et al., 2010).
The effect of CT on microbial protein synthesis and microbial growth efficiency are much less clear compared with the well-established responses on ruminal CP degradation and NH3 concentrations. Addition of QTE at 1, 2, and 3% of DM was reported to improve microbial efficiency in sheep fed M. sativa hay (Al-Dobaib, 2009) and Getachew et al. (2008) reported improvements only at concentrations of 0.5 and 1%, but not 1.5%. In contrast, QTE infusion at 2, 4, or 6% of DM intake reduced duodenal microbial protein flow by 11, 21, and 39% (Dickhoefer et al., 2016) and up to 36% when QTE was supplemented at concentrations greater than 1% of DM intake (Ahnert et al., 2015).
Supplementation with CT-containing forage seems to have similar contradictory responses for different animal species. For dairy cattle, feeding L. corniculatus rather than M. sativa increased microbial protein production (Christensen et al., 2015). In contrast, multiple in vivo sheep trials (n = 11) using a wide range of dietary CT concentrations (0-200 g/kg DM) reported no effect on microbial protein synthesis (Min et al., 2003). Because of the important contribution of microbial protein to ruminant amino acid requirements, future evaluations of CT-containing forages or extracts should address the effects on microbial protein production and efficiency.
Initial interest in utilizing CT to improve ruminant N metabolism was based on work by Waghorn et al. (1987), who reported 50% greater post-ruminal flux of essential amino acids due to L. corniculatus CT followed by an average of 60% improvement in intestinal amino acid availability. The magnitude of these results has not been replicated yet. In general, CT increase post-ruminal amino acid flux due to greater RUP proportions, but effects on intestinal amino acid availability vary widely. Responses seem to depend on CT source and chemical characteristics (Kariuki and Norton, 2008), in addition to diet composition and the physiological state and production level of the animal.
Most CT-protein complexes, depending on the binding affinity, are assumed to dissociate under the acidic conditions of the abomasum, releasing both compounds into the digestion matrix (Patra and Saxena, 2011; Hagerman, 2012). Neutral pH conditions in the small intestine provide another opportunity for CT-nutrient binding, although complexes are less likely to form as the pH increases above neutral (Hagerman et al., 1992). Affinity and binding strength of CT-protein interactions affect protein digestibility throughout the digestive tract.
Kariuki and Norton (2008) directly confirmed the dissociation of proteins from CT post-ruminally in sheep. Bovine serum albumin had greater than 82% true digestibility when introduced through an abomasum cannula as a complex with CT, suggesting the majority of CT-bound protein was released and available postruminally. However, L. corniculatus CT improved postruminal amino acid supply in sheep, but decreased intestinal amino acid availability (Waghorn et al., 1994b). Yet, blood amino acid concentrations indicated the reduced amino acid digestibility was compensated for by the increased N flow to the intestines due to CT; thus, net absorption was not affected (Waghorn et al., 1994b).
Acacia mearnsii tannin extract supplementation of 9-17 g/kg DM to Holstein steers increased amino acid flux into the duodenum by an average of 30% above the mean value for the control treatment (Orlandi et al., 2015). Most importantly for dairy production, methionine and gluconeogenic amino acid supply was positively influenced by CT supplementation. While both apparent and true N digestibility were linearly decreased by 7% or less as CT inclusion increased, N retention was linearly improved from 17.3 to 33.2 g/day (Orlandi et al., 2015). This finding suggests that an improvement in post-ruminal amino acid supply outweighed any impairment in protein digestibility. These data also provide some insight into CT effects on endogenous protein secretion.
Increased endogenous protein losses, primarily through increased mucus production (Sell et al., 1984) or CT complexation with digestive enzymes (Al-Mamary et al., 2001), is an often cited explanation for observed impairments in intestinal protein digestibility in both ruminants (Waghorn, 2008) and non-ruminants (Jansman, 1993). Orlandi et al. (2015) reported a similar degree of N digestibility inhibition when calculated on an apparent basis and when the effect of endogenous losses was removed by utilizing only neutral detergent insoluble N as a proxy for N of dietary origin. This suggests that Acacia mearnsii tannins at concentrations of 0.9-1.7% of DM did not stimulate endogenous protein secretion. Whether CT increase endogenous protein secretion apparently decreasing total tract protein digestibility, overall N retention and animal performance is the most important metric by which to evaluate CT supplementation effectiveness.
The fate of CT in the context of hypothesized CT-protein interactions remains unclear. What happens to the potentially stable CT-protein complexes as they translocate from the rumen to the abomasum and beyond? One hypothesis is that CT are depolymerized by acid hydrolysis of interflavan bonds at acidic pH encountered in the abomasum. However, if CT are not degraded in the rumen or abomasum, it is possible that CT could re-complex with any undigested proteins, peptides, or amino acids, thereby preventing absorption of these compounds in the small intestine and reducing a potential increase in amino acid absorption by the animal. This could be a possible explanation for a shift away from urinary N excretion (Grainger et al., 2009) and a decrease in urease activity and subsequent ammonia volatilization from feces (Aguerre et al., 2011) when ruminants are consuming a diet rich in CT.
In vivo experiments can help answer more practical questions relating to CT efficacy in animal production. Fully grown heifers fed a grass hay and concentrate diet with QTE added at 1-6% of the daily DM intake had reduced apparent total tract CP digestibility at dosages greater than 2%, but impaired digestibility of other nutrients when QTE was greater than 4% (Ahnert et al., 2015). Despite the negative digestibility effect, QTE inclusion improved overall N retention compared with the control, irrespective of the dosage level. This result suggests muscle growth may not be impaired as long as adequate levels of digestible energy are available. Similar responses on total tract nutrient digestibility were observed in lactating dairy Holstein cows by Aguerre et al. (2016), who reported linear digestibility decreases due to inclusion of a tannin mixture (1/3 chestnut extract, 2/3 quebracho extract) at 0.45, 0.90, and 1.80% of DM. Milk production efficiency (kg milk/DMI) was improved, but milk protein yield (kg true protein/day) decreased linearly.
Animal agriculture must efficiently utilize feed N and prevent excessive N release to the environment to achieve and maintain sustainability. Condensed tannins are a potential tool for nutritionists to achieve this goal. Although clarification is needed for CT effects on post-ruminal amino acid availability and endogenous N secretion, it is well accepted that low to moderate dietary CT concentrations slow CP degradation in the rumen resulting in increased proportions of RUP and greater post-ruminal amino acid flux. Conservatively assuming the improvement in intestinal amino acid abundance is negated by decreased apparent protein digestibility, improvements in ruminant N balance and physiology seem to be due primarily to reductions in rumen NH3 concentrations, which consequently diminish metabolic costs associated with urinary urea excretion and inefficient N recycling. Furthermore, CT represent an opportunity to manipulate dietary RUP and target a profile of post-ruminal amino acids to more closely match requirements of animals and improve beef and dairy production efficiency.
The anthelmintic activity of condensed tannins
One of the greatest concerns of ruminant producers is that of gastrointestinal nematode parasites, especially Haemonchus contortus. Major costs associated with these infections include treatment with anthelmintic pharmaceuticals, losses from decreased animal production, and losses due to animal death, all exacerbated by the increasing anthelmintic resistance of parasite populations (Leathwick and Besier, 2014). Offering forages or feeds that contain CT to ruminants has potential for mitigating the gastrointestinal parasite problem. Supplementation of Lespedeza cuneata at 50 and 75% of the DM decreased fecal egg count, specifically of Haemonchus contortus, by 84.6 and 91.9% in Boer goats (Terrill et al., 2009). Minho et al. (2008) reported reductions in fecal egg count and adult Haemonchus contortus in the abomasum of sheep when CT extracts of Acacia molissima (15% CT, DM basis) was given, but no effect was observed on adult Trichostrongylus colubriformis in the small intestines. The anthelmintic properties of CT may not only be parasite specific, but might also be compartment specific (Tedeschi et al., 2014): some have reported greater effectiveness of CT against gastrointestinal nematode parasites in the abomasum rather than in the small intestine. A major challenge of using CT-containing plants as anthelmintics is that not all forages containing CT have anthelmintic properties (Naumann et al., 2014a). Condensed tannins from Leucaena retusa, Lespedeza stuevei, and Acacia angustissima var. hirta decreased larval migration of Haemonchus contortus by 65.4, 63.1, and 42.2%, respectively. Condensed tannins from Desmanthus illinoensis, Lespedeza cuneata, and A. angustissima were less effective against L3 larvae. Like CT-protein interactions, variation in anthelmintic activity in response to different sources of CT is probably a structure-function relationship. Quijada et al. (2015) demonstrated that CT composed predominately of prodelphinidin subunits had greater anthelmintic activity than those composed predominantly of procyanidin. This may explain why L. cuneata, a forage that produces CT composed predominately of prodelphinidin subunits (Naumann et al., 2015b), has demonstrated high anthelmintic efficacy in vivo (Shaik et al., 2006; Terrill et al., 2007; Terrill et al., 2009). Similar findings have been reported with cattle parasites. Condensed tannins composed predominantly of prodelphinidin reduced motility of L1 and adult stage Ostertagia ostertagi and Cooperia oncophora (Desrues et al., 2016). There is enough evidence that smaller CT polymers have greater anthelmintic activity than large CT polymers (Naumann et al., 2014a, Barrau et al., 2005). However, Quijada et al. (2015) and Desrues et al. (2016) reported the opposite effect and suggested that variation in results is likely due to differences and difficulties in extraction and purification of CT, as well as differences in modes of action of different CT on different gastrointestinal nematode parasites. The mode of action of CT on gastrointestinal nematode parasites has not been entirely clarified. However, electron micrographs of nematode larvae following incubation with CT extract show alteration of the nematode cuticle and binding of CT in the cephalic region (Hoste et al., 2012). Condensed tannins in combination with other plant specialized metabolites may impact the efficacy of these compounds as anthelmintics. Klongsiriwet et al. (2015) demonstrated synergistic effects of combining flavonoids such as quercitin and luteolin with CT. The anthelmintic activity of CT was enhanced in the presence of flavonoids using an in vitro larval exsheathment assay. Using HT may also have promise as a potential anthelmintic. Engström et al. (2016) demonstrated that many HT have little if any anthelmintic efficacy. However, those composed primarily of the PGG structure demonstrated inhibition of egg hatching and larval motility. Evidence suggests that the external structure of the egg, larval midbody, and cephalic region are altered in the presence of HT (Engström et al., 2016).The interactions between minerals and condensed tannins
In addition to the ability to bind to and precipitate proteins, tannins and other polyphenols efficiently bind to Fe and, to a lesser extent, to Cu, Mn, Al, Zn, and Co. There is great variability in the extent to which polyphenol-metal chelation affects mineral bioavailability. Yet, evaluations of CT supplementation in both ruminants and monogastrics rarely address the potential effects on mineral nutrition. While CT effects on mineral availability in vivo remain vague, the chemistry behind the phenomenon is better understood. The presence of at least two adjacent hydroxyl groups on a phenyl ring (ortho or o-dihydroxyphenyl group) are the minimum requirements for mineral chelation (Andjelkovic et al., 2006). This structural chemistry contributes to plant pigmentation as well as cation-nutrient cycling throughout the environment of the plants (Quideau et al., 2011). To generalize, the more o-dihydroxyphenyl functional groups in a CT molecule, the greater potential for metal chelation (McDonald et al., 1996). The interaction is pH dependent and greatly influences CT anti-oxidative capacity (Kumamoto et al., 2001). Iron and Al cations bound to CT at pH levels of 3.20 or less, whereas Mg, Ca, Zn, Cu, Mn, and Co cations bound to CT at a pH greater than 3.70 (Faithfull, 1984). However, it is generally accepted that these complexes are stable over a wide pH range and throughout the entire gastrointestinal tract (McDonald et al., 1996; Kumamoto et al., 2001; Scalbert et al., 2002). Iron is the most frequently studied mineral in relation to tannins due to strong Fe-binding potential. Early studies investigating iron absorption inhibition by phenolic compounds indicated a close relationship between the amount of tannin added to a meal and the degree of inhibition (Brune et al., 1989). Since then, functional groups important for iron and other mineral binding have been identified. Tannins with catechol and galloyl groups are effective metal chelators (Andjelkovic et al., 2006; Perron and Brumaghim, 2009). Each CT molecule may bind two or more metal ions and each metal ion may form chelates with o-dihydroxyphenyl groups from two different CT molecules (McDonald et al., 1996). A 3’,4’-dihydroxygroup on a flavonoid B-ring (Figure 6) is required for Fe binding (Khokhar and Owusu-Apenten, 2003) and increased free hydroxyl groups are associated with increased Fe-binding ability (Andjelkovic et al., 2006; Mladénka et al., 2011).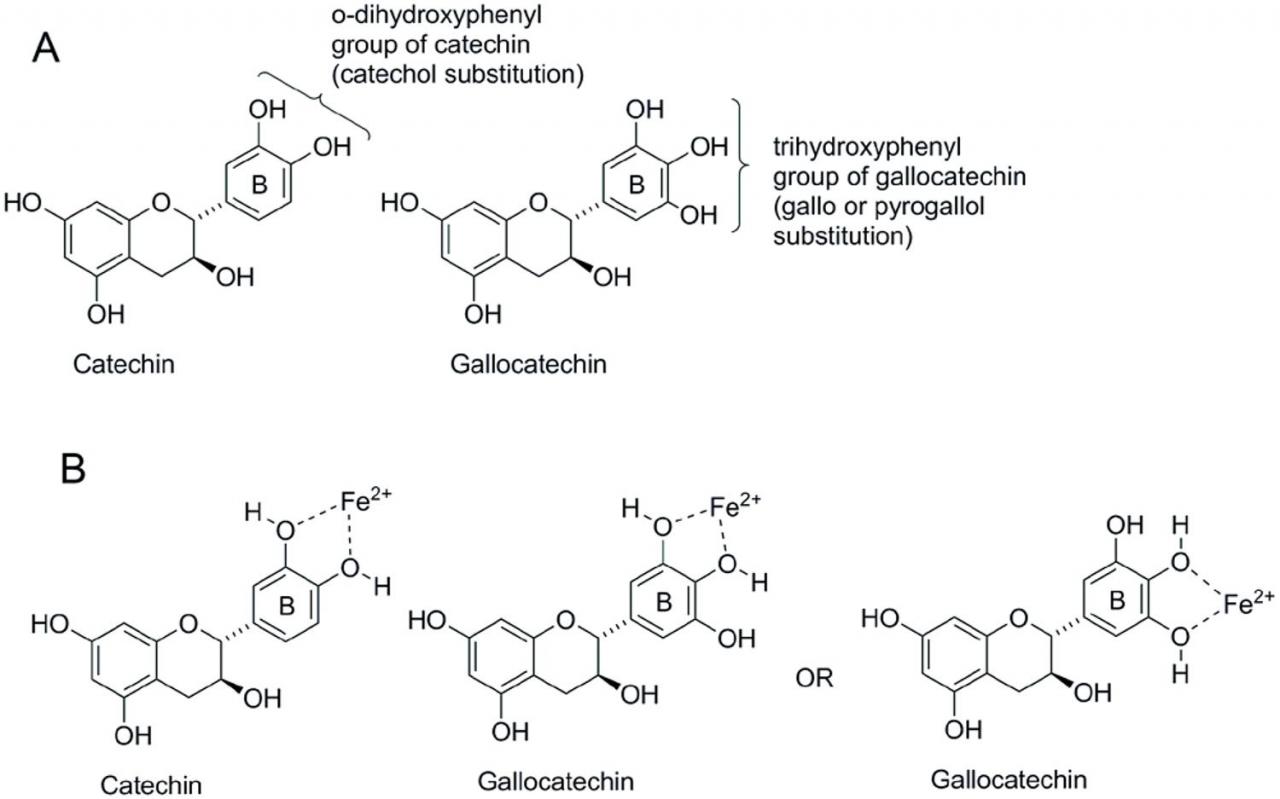
Figure 6 Phenolic hydroxyl substitution patterns for catechin and gallocatechin (A) and phenolic hydroxyl-ferrous iron binding modes for catechin and gallocatechin (B).
CT-enteric fermentation interactions
Methane is a potent greenhouse gas that is produced normally during microbial fermentation in the rumen and released to the environment during eructation. Methane gas produced by livestock represents the second greatest source of CH4 to the atmosphere, estimated at 22% of total anthropogenic CH4 emissions in the US; beef and dairy cattle are responsible for 96% of these emissions (USEPA, 2016). However, when expressed as CO2 equivalents, the livestock sector is responsible for 4.2% of total greenhouse gas emissions; beef and dairy cattle are the primary drivers of CH4 production from the livestock sector, representing only 3.6% of the total contribution (USEPA, 2016). Methanogenesis is a complex process by which methanogens in the rumen digest cellulose into forms usable by the animal. Buddle et al. (2011) developed a simplified diagrammatic representation of this process in which rumen bacteria, protozoa, and fungi act upon feedstuffs entering the rumen. Metabolic byproducts from rumen microbes include H2, CO2, and volatile fatty acids (VFA), among others. Enteric CH4 is produced during the disposal of metabolic H2. Reducing equivalents that are not consumed during the formation of volatile fatty acids may be used to produce CH4, representing a loss of metabolizable energy to the animal. One of the many important symbiotic associations formed in the rumen is that of the relationship between methanogenic archaea and ciliated protozoa (Ng et al., 2016). It has been proposed that this association occurs to facilitate interspecies transfer of metabolic H2 from protozoa to methanogens. Carbon dioxide is reduced by methanogens to produce energy, creating CH4 as a metabolic byproduct in the following way: 4H2 + CO2 → CH4 + 2H2O. It is also postulated that H2 in combination with CO2 could be utilized by homoacetogens to produce acetate, a primary energy source for the ruminant animal. Thus, if less H2 is converted to CH4, then more H2 is available for VFA production, resulting in an increase in metabolizable energy for the animal. Feeding CT-containing forages or feedstuffs to ruminants may be an effective natural practice for mitigating CH4 emissions by ruminant livestock and increasing metabolizable energy intake. A diverse group of legume species with varying types of CT inhibited CH4 production in vitro (Naumann et al., 2013b). However, when these CT-containing legumes were fermented as the sole source of forage, they also inhibited total gas production and VFA production, indicating an inhibition of digestibility. This suggests that inhibiting CH4 does not result in a shift in H2 from methanogens and methanogenesis to homoacetogens and VFA production and demonstrates a downside of using CT for CH4 mitigation. The objective should be to decrease CH4 emissions from ruminant livestock without compromising production, which requires selectively reducing CH4 production without subsequent reductions in total gas or fermentation. It is possible to accomplish this objective with CT. In in vitro rumen fermentation studies, replacement of 45% of the forage portion of a corn-alfalfa diet for either L. cuneata or D. paniculatum, achieving dietary CT concentrations of 2.6 and 9%, respectively, decreased CH4 production without decreasing total gas production (Naumann et al., 2015b). The mechanism by which CT impact methanogenesis and reduces CH4 production by ruminants is not well understood. There are multiple hypotheses for how CT inhibit methanogenesis, none of which have been definitively proven. One hypothesis is that CT act directly upon methanogens in the rumen. Ng et al. (2016) reported the existence of a protein-based adhesin probably located at the tips of fimbriae that function in facilitation of the methanogen-protozoa symbiosis. Parts of the cell envelope, including the cell membrane, wall, and glycocalyx, also contain protein. It is possible that CT bind to this proteinaceous adhesin or parts of the cell envelope, interfering with establishment of the methanogen-protozoa complex and decreasing interspecies H2 transfer. Inhibition of this symbiotic relationship may also negatively impact the ciliated protozoa population. Bhatta et al. (2015) determined that rumen ciliated protozoa populations decreased when the feedstuff contained CT from Ficus bengalensis and Azardirachta indica at concentrations of 26 and 13.8%, respectively. Another hypothesis is that indirect inhibition occurs by decreasing the availability of nutrients to rumen microorganisms, subsequently reducing substrate digestibility and indirectly inhibiting rumen microbial populations. Because CT bind to minerals (Lavin, 2012) and organic molecules such as proteins (Saminathan et al., 2014), carbohydrates (Soares et al., 2012a), and lipids (Delehanty et al., 2007), it is possible that not only do these complexes become unavailable as substrate for use by rumen microbes, but that CT bind to microbial enzymes modulating their activity (Gonçalves et al., 2011). Naumann et al. (2013c) demonstrated a weak relationship between protein bound by CT and a decrease in CH4. A third hypothesis for how CT inhibit CH4 is that CT act as a hydrogen sink (Naumann et al., 2013a). Becker et al. (2014) reported an abatement of methane production in an in vitro rumen fluid environment that occurs linearly with the addition of flavan-3-ol catechin. In this experiment, as many as six hydrogen atoms per catechin molecule were captured by catechin-degradation products and CH4 production was reduced at a rate of 1.2 mol CH4 per mol catechin. From this study, the authors reported characterization of metabolism products arising from catechin acting as a hydrogen atom acceptor (hydrogen sink). These findings parallel compounds isolated from biotransformations of flavan-3-ols by human microflora (Feng, 2006; Monagas et al., 2010). Briefly, catechin is converted to transient intermediate 2, which accepts two hydrogen atoms to provide reduced compound 3 (Figure 7). Compound 3 accepts an additional two hydrogen atoms as hydrogenolysis of the C-4 phenolic bond occurs, reducing it to a C-H bond and giving rise to compound 4. Compound 5 can be derived from transient intermediate 2 through scission of the A-ring, leading to excision of a molecule of acetoacetic acid. Compound 5 accepts two more hydrogen atom equivalents undergoing dehydroxylation of the C-4 phenolic group of the B-ring to provide compound 6. Compound 3 can undergo A-ring scission, from which the intermediate hydroxyacid undergoes lactonization to deliver compound 7. Alternatively, reduction of the ketone functionality in compound 5 leads to the same intermediate hydroxyacid as derived from 3 and provides lactone 7. Hydrogenolysis, requiring two hydrogen atom equivalents, of the C-O bond of the lactone in 7 provides phenolic acid 8. Hydrogenolysis of the C-4 phenolic bond in compound 8, again requiring two hydrogen atom equivalents, provides phenolic acid 9. Although intermediates were not detected in this study to support its subsequent transformation to compound 9, compound 4 presumably could undergo biotransformations similar to those proposed for the conversion of compound 3 to compound 9 (A-ring scission/lactonization, lactone hydrogenolysis) to deliver compound 9. Lastly, compound 6 could presumably be converted to compound 9 through a reduction/lactonization followed by lactone hydrogenolysis sequence. All structures (Figure 7), with the exception of compound 2, were either confirmed through comparison with commercially available materials or characterized by mass spectrometry and nuclear magnetic resonance spectroscopy.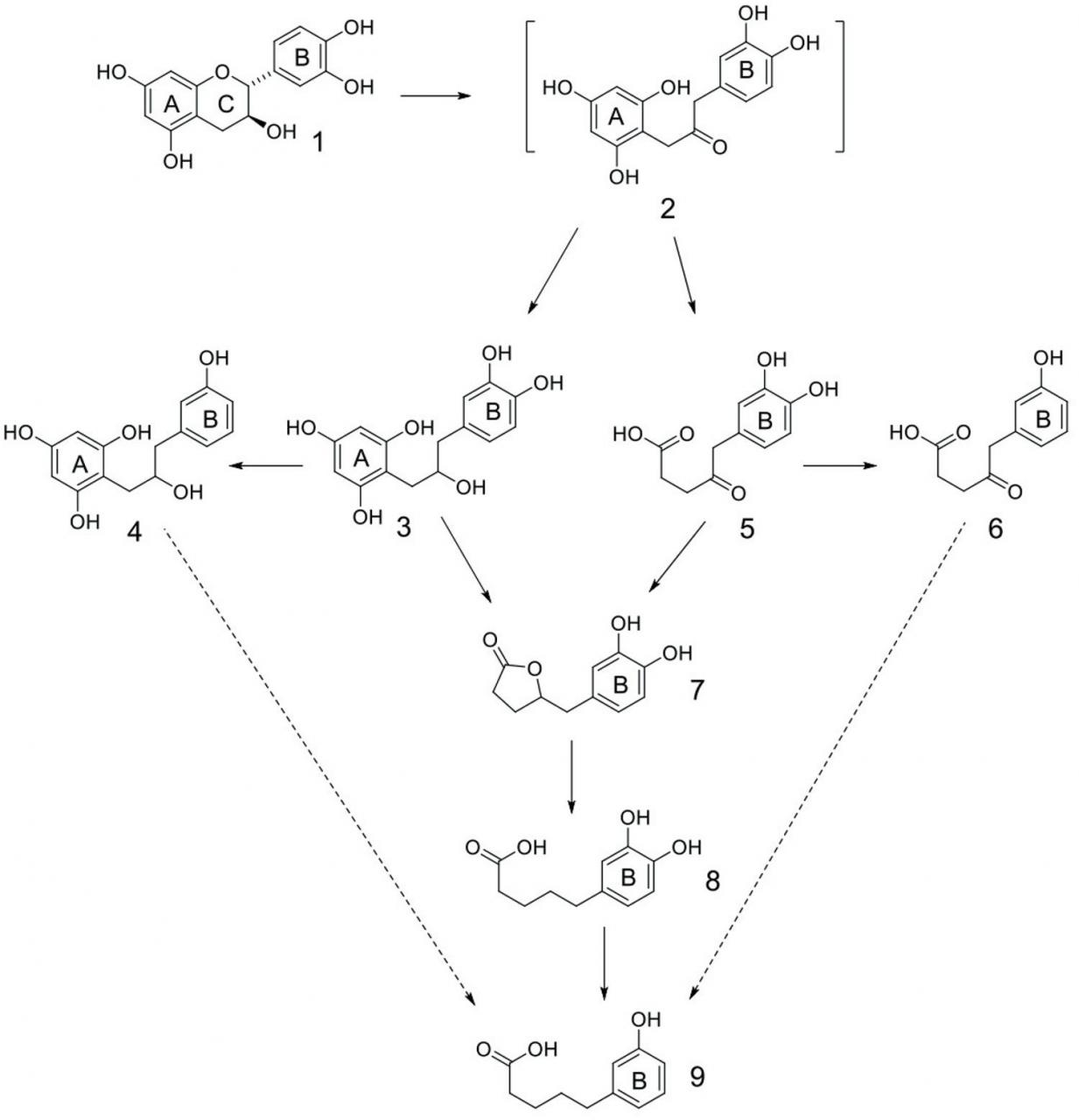
Figure 7 Catechin as a hydrogen sink during in vitro rumen fermentation. Adapted from Becker et al. (2014).
Limitations and future directions
Probably, the greatest limitation in advancing knowledge of CT-animal interactions is related to the structural diversity of CT and its chemical determination. As described above, CT are assembled through the joining of a subset of flavan-3-ol subunits through a few different covalent bonding patterns. So, how many unique chemical entities (isomers) can be derived from a small collection of flavan-3-ol subunits and a defined set of interflavan linkages? Calculation of the number of potential isomers in a CT oligomer/polymer (Table 1) was performed using the equation (Am × Bn) = number of possible isomers, in which A = number of different flavan-3-ol subunit types in the compound, m = the actual number of flavan-3-ol subunits in the compound, B = the number of different types of interflavan linkages present in the compound, and n = the actual number of interflavan linkages present in the compound. Similar formulas have been reported to determine the number of CT structural isomers (Cheynier, 2005; Nam et al., 2017). The number of different unique compounds that could arise from CT containing two different flavan-3-ol subunits and only two types of interflavan linkages is listed in column A (Table 1). The CT from Vaccinium (cranberry) and Sorghum would fall into the category of compounds listed in this column for chemical structural isomers. Vaccinium and Sorghum CT are almost exclusively composed of procyanidin (catechin and epicatechin) subunits. Both Sorghum and Vaccinium CT possess 4-8 B-type interflavan linkages. Sorghum may also contain 4-6 B-type linkages and cranberries are known to contain A-type interflavan linkages. The CT from common forages such as L. corniculatus, L. pedunculatus, and O. viciifolia contain a varying mixture of PC and PD subunits, increasing the complexity of the CT (Table 1, Columns E and F). To add to the complexity, CT structures isolated from some sources, such as Vitis or Diospyros (Tian et al. 2012), are found to contain a derivatized C3 hydroxyl group as the gallate ester. This may occur multiple times and at any point along the oligomer/polymer chain. The numbers listed in Table 1 also do not take into account that occasionally enantiomers (mirror images) of flavan-3-ol subunits are isolated and characterized. Both of these occurrences would greatly increase the number of possible structural isomers and add to the challenge of identifying and characterizing CT from plant materials.Table 1 Number of possible condensed tannin isomers dependent on number of unique flavan-3-ol subunits and interflavan bond types present
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Number of flavan-3-ol units | Two units Two bond types | Two units Three bond types | Three units Two bond types | Three units Three bond types | Four units Two bond types | Four units Three bond types |
| 2 (dimer) | 8 | 12 | 18 | 27 | 32 | 48 |
| 3 (trimer) | 32 | 72 | 108 | 243 | 256 | 576 |
| 4 (tetramer) | 128 | 432 | 648 | 2187 | 2048 | 6,912 |
| 5 (pentamer) | 512 | 2592 | 3888 | 19,683 | 16,384 | 82,944 |
| 6 (hexamer) | 2048 | 15,552 | 23,328 | 177,147 | 131,072 | 995,328 |
| 7 (heptamer) | 8192 | 93,312 | 139,968 | 1,594,323 | 1,048,576 | 11,943,936 |
| 8 (octamer) | 32,768 | 559,872 | 839,808 | 14,348,907 | 8,388,608 | 143,327,232 |
| 9 (nonamer) | 131,072 | 3,359,232 | 5,038,848 | 129,140,163 | 67,108,864 | 1,719,926,784 |
| 10 (decamer) | 524,288 | 20,155,392 | 30,233,088 | 1,162,261,467 | 536,870,912 | 20,639,121,408 |
ACKNOWLEDGMENTS
The authors would like to acknowledge UNESP40, “1st International Meeting of Advances in Animal Science”, Jaboticabal, São Paulo, Brazil. Mention of trade names or commercial products in this article is solely to provide specific information and does not imply recommendation or endorsement by the US Department of Agriculture.concentration, protein/tannin ratio and pH. International Journal of Food Science and Technology 47:875-878. [ Links ] Afsana, K.; Shiga, K.; Ishizuka, S. and Hara, H. 2004. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Bioscience, Biotechnology and Biochemistry 68:584-592. [ Links ] Agle, M.; Hristov, A. N.; Zaman, S.; Schneider, C.; Ndegwa, P. and Vaddella, V. K. 2010. The effects of ruminally degraded protein on rumen fermentation and ammonia losses from manure in dairy cows. Journal of Dairy Science 93:1625-1637. [ Links ] Aguerre, M. J.; Wattiaux, M. A.; Powell, J. M.; Broderick, G. A. and Arndt, C. 2011. Effect of forage-to-concentrate ratio in dairy cow diets on emission of methane, carbon dioxide, and ammonia, lactation performance, and manure excretion. Journal of Dairy Science 94:3081-3093. [ Links ] Aguerre, M. J.; Capozzolo, M. C.; Lencioni, P.; Cabral, C. and Wattiaux, M. A. 2016. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation and nitrogen partitioning in dairy cows. Journal of Dairy Science 99:4476-4486. [ Links ] Ahnert, S.; Dickhoefer, U.; Schulz, F. and Susenbeth, A. 2015. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livestock Science 177:63-70. [ Links ] Al-Dobaib, S. N. 2009. Effect of different levels of Quebracho tannin on nitrogen utilization and growth performance of Najdi sheep fed alfalfa (Medicago sativa) hay as a sole diet. Animal Science Journal 80:532-541. [ Links ] Al-Mamary, M.; Molham, A. H.; Abdulwali, A. A. and Al-Obeidi, A. 2001. In vivo effects of dietary sorghum tannins on rabbit digestive enzymes and mineral absorption. Nutrition Research 21:1393-1401. [ Links ] Alonso-Diaz, M. A.; Torres-Acosta, J. F. J.; Sandoval-Castro, C. A. and Capetillo-Leal, C. M. 2012. Amino acid profile of the protein from whole saliva of goats and sheep and its interaction with tannic acid and tannins extracted from the fodder of tropical plants. Small Ruminant Research 103:69-74. [ Links ] Anderson, R. A.; Broadhurst, C. L.; Polansky, M. M.; Schmidt, W. F.; Khan, A,; Flanagan, V. P.; Schoene, N. W. and Graves, D. J. 2004. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. Journal of Agricultural and Food Chemistry 52:65-70. [ Links ] Andjelkovic, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M. and Verhe, R. 2006. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chemistry 98:23-31. [ Links ] Barrau, E.; Fabre, N.; Fouraste, I. and Hoste, H. 2005. Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: role of tannins and flavonol glycosides. Parasitology 131:531-538. [ Links ] Becker, P. M.; Wikselaar, P. G.; Franssen, M. C. R.; Vos, R. C. H.; Hall, R. D. and Beekwilder, J. 2014. Evidence for a hydrogen-sink mechanism of (+)catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 10:179-189. [ Links ] Bhatta, R.; Saravanan, M.; Baruah, L. and Prasad, C. S. 2015. Effects of graded levels of tannin-containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. Journal of Applied Microbiology 118:557-564. [ Links ] Brenes, A.; Viveros, A.; Goni, I.; Centeno, C.; Sáyago-Ayerdy, S. G.; Arija, I. and Saura-Calixto, F. 2008. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poultry Science 87:307-316. [ Links ] Brooks, M. A.; Harvey, R. M.; Johnson, N. F. and Kerley, M. S. 2012. Rumen degradable protein supply affects microbial efficiency in continuous culture and growth in steers. Journal of Animal Science 90:4985-4994. [ Links ] Brune, M.; Rossander, L. and Hallberg, L. 1989. Iron absorption and phenolic compounds: importance of different phenolic structures. European Journal of Clinical Nutrition 43:547-558. [ Links ] Bruno-Soares, A. M.; Soares-Pereira, A. L.; Matos, T. J. S. and Ricardo-da-Silva, J. M. 2011. Preliminary results on the effects of grape (Vitis vinifera) seed condensed tannins on in vitro intestinal digestibility of the lupin (Lupinus angustifolius) seed protein fraction in small ruminants. Journal of Animal Physiology and Animal Nutrition 95:456-460. [ Links ] Buddle, B. M.; Denis, M.; Attwood, G. T.; Altermann, E.; Janssen, P. H.; Ronimus, R. S.; Pinares-Patino, C. S.; Muetzel, S. and Neil Wedlock, D. 2011. Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Veterinary Journal 188:11-17. [ Links ] Buzzini, P., Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F. and Romani, A. 2008. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Reviews in Medicinal Chemistry 8:1179-1187. [ Links ] Calsamiglia, S.; Ferret, A.; Reynolds, C. K.; Kristensen, N. B. and van Vuuren, A. M. 2010. Strategies for optimizing nitrogen use by ruminants. Animal 4:1184-1196. [ Links ] Canon, F.; Ballivian, R.; Chirot, F.; Antoine, R.; Sarni-Manchado, P.; Lemoine, J. and Dugourd, P. 2011. Folding of a salivary intrinsically disordered protein upon binding to tannins. Journal of the American Chemical Society 133:7847-7852. [ Links ] Cappai, M. G.; Wolf, P.; Pinna, W. and Kamphues, J. 2013. Pigs use endogenous proline to cope with acorn (Quercus pubescens Willd.) combined diets high in hydrolysable tannins. Livestock Science 155:316-322. [ Links ] Carnovale, V.; Britten, M.; Couillard, C. and Bazinet, L. 2015. Impact of calcium on the interactions between epigallocatechin-3-gallate and β-lactoglobulin. Food Research Interntational 77:565-571. [ Links ] Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I. and Brenes, A. 2013. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 7:555-561. [ Links ] Charlton, A. J.; Baxter, N. J.; Khan, M. L.; Moir, A. J. G.; Haslam, E.; Davies, A. P. and Williamson, M. P. 2002. Polyphenol/peptide binding and precipitation. Journal of Agricultural and Food Chemistry 50:1593-1601. [ Links ] Cheynier, V. 2005. Polyphenols in foods are more complex than often thought. American Society for Clinical Nutrition 81:223S-229S. [ Links ] Christensen, R. G.; Yang, S. Y.; Eun, J.-S.; Young, A. J.; Hall, J. O. and MacAdam, J. W. 2015. Effects of feeding birdsfoot trefoil hay on neutral detergent fiber digestion, nitrogen utilization efficiency, and lactational performance by dairy cows. Journal of Dairy Science 98:7982-7992. [ Links ] Coblentz, W. K. and Grabber, J. H. 2013. In situ protein degradation of alfalfa and birdsfoot trefoil hays and silages as influenced by condensed tannin concentration. Journal of Dairy Science 96:3120-3137. [ Links ] de Freitas, V. and Mateus, N. 2002. Nephelometric study of salivary protein-tannin aggregates. Journal of the Science of Food and Agriculture 82:113-119. [ Links ] Deaville, E. R.; Givens, D. I. and Mueller-Harvey, I. 2010. Chestnut and mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilisation and losses. Animal Feed Science and Technology 157:129-138. [ Links ] Delehanty, J. B.; Johnson, B. J.; Hickey, T. E.; Pons, T. and Ligler, F. S. 2007. Binding and neutralization of lipopolysaccharides by plant proanthocyanidins. Journal of Natural Products 70:1718-1724. [ Links ] Desrues, O.; Fryganas, C.; Ropiak, H. M.; Mueller-Harvey, I.; Enemark, H. L. and Thamsborg, S. M. 2016. Impact of chemical structure of flavanol monomers and condensed tannins on in vitro anthelmintic activity against bovine nematodes. Parasitology 143:444-454. [ Links ] Dickhoefer, U.; Ahnert, S. and Susenbeth, A. 2016. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. Journal of Animal Science 94:1561-1575. [ Links ] Dittmann, M. T.; Runge, U.; Lang, R. A.; Moser D.; Galeffi, C.; Kreuzer, M. and Clauss, M. 2014. Methane emission by camelids. PLoS One 9:1-9. [ Links ] Dobreva, M. A.; Frazier, R. A.; Mueller-Harvey, I.; Clifton, L. A.; Gea, A. and Green, R. J. 2011. Binding of pentagalloyl glucose to two globular proteins occurs via multiple surface sites. Biomacromolecules 12:710-715. [ Links ] Doce, R. R.; Belenguer, A.; Toral, P. G.; Hervás, G. and Frutos, P. 2013. Effect of the administration of young leaves of Quercus pyrenaica on rumen fermentation in relation to oak tannin toxicosis in cattle. Journal of Animal Physiology and Animal Nutrition 97:48-57. [ Links ] Dschaak, C. M.; Williams, C. M.; Holt, M. S.; Eun, J.-S.; Young, A. J. and Min, B. R. 2011. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. Journal of Dairy Science 94:2508-2519. [ Links ] Elrod, C. C. and Butler, W. R. 1993. Reduction of fertility and alteration of uterine pH in heifers fed excess ruminally degradable protein. Journal of Animal Science 71:694-701. [ Links ] Engström, M. T.; Karonen, M.; Ahern, J. R.; Baert, N.; Payré, B.; Hoste, H. and Salminen, J. P. 2016. Chemical structures of plant hydrolyzable tannins reveal their in vitro activity against egg hatching and motility of Haemonchus contortus Nematodes. Journal of Agriculture and Food Chemistry 64:840-851. [ Links ] Fairweather-Tait, S.; Piper, Z.; Fatemi, S. J. A. and Moore, G. R. 1991. The effect of tea on iron and aluminium metabolism in the rat. British Journal of Nutrition 65:61-68. [ Links ] Faithfull, N. T. 1984. The in-vitro digestibility of feedstuffs-a century of ferment. Journal of the Science of Food and Agriculture. 35:819-826. [ Links ] Feng, W. Y. 2006. Metabolism of green tea catechins: An overview. Current Drug Metabolism 7:755-809. [ Links ] Filippich, L. J.; Zhu, J.; Oelrichs, P.; Alsalami, M. T.; Doig, A. J.; Cao, G. R. and English, P. B. 1991. Hepatotoxic and nephrotoxic principles in Terminalia oblongata. Research in Veterinary Science 50:170-177. [ Links ] Foo, L. Y.; Lu, Y.; Howell, A. B. and Vorsa, N. 2000. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic p-fimbriated Escherichia coli. Journal of Natural Products 63:1225-1228. [ Links ] Getachew, G.; Pittroff, W.; Putnam, D. H.; Dandekar, A.; Goyal, S. and Depeters, E. J. 2008. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Animal Feed Science and Technology 140:444-461. [ Links ] Glahn, R. P. and Wortley, G. M. 2002. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an in vitro digestion/Caco-2 cell model. Journal of Agriculture and Food Chemistry 50:390-395. [ Links ] Gonçalves, R.; Mateus, N. and De Freitas, V. 2011. Inhibition of alpha-amylase activity by condensed tannins. Food Chemistry 125:665-672. [ Links ] Grainger, C. and Beauchemin, K. A. 2011. Can enteric methane emissions from ruminants be lowered without lowering their production? Animal Feed Science and Technology 166-167:308-320. [ Links ] Grainger, C.; Clarke, T.; Auldist, M. J.; Beauchemin, K. A.; McGinn, S. M.; Waghorn, G. C. and Eckard, R. J. 2009. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Canadian Journal of Animal Science 89:241-251. [ Links ] Greger, J. L. and Lyle, B. J. 1988. Iron, copper and zinc metabolism of rats fed various levels and types of tea. Journal of Nutrition 118:52-60. [ Links ] Hagerman, A. E. and Butler, L. G. 1978. Protein precipitation method for the quantitative determination of tannins. Journal of Agriculture and Food Chemistry 26:809-812. [ Links ] Hagerman, A. E. and Butler, L. G. 1981. The specificity of proanthocyanidin-protein interactions. Journal of Biological Chemistry 256:4494-4497. [ Links ] Hagerman, A. E.; Robbins, C. T.; Weerasuriya, Y.; Wilson, T. C. and McArthur, C. 1992. Tannin chemistry in relation to digestion. Journal of Range Management 45:57-62. [ Links ] Hagerman, A. E. and Robbins, C. T. 1993. Specificity of tanninbinding salivary proteins relative to diet selection by mammals. Canadian Journal of Zoology 71:628-633. [ Links ] Hagerman, A. E.; Rice, M. E. and Ritchard, N. T. 1998. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16 (4-8) catechin (procyanidin). Journal of Agricultural and Food Chemistry 46:2590-2595. [ Links ] Hagerman, A. E. 2011. Tannin handbook. Miami University, Oxford, Ohio. Available at: <http://www.users.muohio.edu/hagermae/>. Accessed on: June 27, 2016. [ Links ] Hagerman, A. E. 2012. Fifty years of polyphenol-protein complexes. p.71-97. In: Recent advances in polyphenol research. vol. 3. 3rd ed. Cheynier, V.; Sarni-Manchado, P. and Quideau, eds. John Wiley & Sons, Ltd., Oxford, UK. [ Links ] Haring, D. A.; Scharenberg, A.; Heckendorn, F.; Dohme, F.; Luscher, A.; Maurer, V.; Suter, D. and Hertzberg, H. 2008. Tanniferous forage plants: Agronomic performance, palatability and efficacy against parasitic nematodes in sheep. Renewable Agriculture and Food Systems 23:19-29. [ Links ] Hassan, I. A.; Elzubeir, E. A. and El Tinay, A. H. 2003. Growth and apparent absorption of minerals in broiler chicks fed diets with low or high tannin contents. Tropical Animal Health and Production 35:189-196. [ Links ] Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J. F. J. and Sandoval-Castro, C. A. 2012. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Veterinary Parasitology 186:18-27. [ Links ] Hymes-Fecht, U. C.; Broderick, G. A.; Muck, R. E. and Grabber, J. H. 2013. Replacing alfalfa or red clover silage with birdsfoot trefoil silage in total mixed rations increases production of lactating dairy cows. Journal of Dairy Science 96:460-469. [ Links ] Jansman, A. J. M. 1993. Tannins in feedstuffs for simple-stomached animals. Nutrition Research Reviews 6:209-236. [ Links ] Jayanegara, A.; Goel, G.; Makkar, H. P. S. and Becker, K. 2015. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Animal Feed Science and Technology 209:60-68. [ Links ] Jin, L.; Wang, Y.; Iwaasa, A. D.; Xu, Z.; Schellenberg, M. P.; Zhang, Y. G.; Liu, X. L. and McAllister, T. A. 2012. Effect of condensed tannins on ruminal degradability of purple prairie clover (Dalea purpurea Vent.) harvested at two growth stages. Animal Feed Science and Technology 176:17-25. [ Links ] Johnson, N. F.; Lees, M. E.; Kerley, M. S. and Naumann, H. D. 2015. Effect of tannin-containing legume forages on crude protein degradation in vitro. Journal of Animal Science 93(suppl. 2):97. [ Links ] Kariuki, I. and Norton, B. 2008. The digestion of dietary protein bound by condensed tannins in the gastro-intestinal tract of sheep. Animal Feed Science and Technology 142:197-209. [ Links ] Khokhar, S. and Owusu-Apenten, R. K. 2003. Iron binding characteristics of phenolic compounds: some tentative structure-activity relations. Food Chemistry 81:133-140. [ Links ] Klongsiriwet, C.; Quijada, J.; Williams, A. R.; Mueller-Harvey, I.; Williamson, E. M. and Hoste, H. 2015. Synergistic inhibition of haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. International Journal of Parasitology Drugs and Drug Resistance 5:127-134. [ Links ] Kohn, R. A.; Dinneen, M. M. and Russek-Cohen, E. 2005. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. Journal of Animal Science 83:879-889. [ Links ] Kumamoto, M.; Sonda, T.; Nagayama, K. and Tabata, M. 2001. Effects of pH and metal ions on antioxidative activities of catechins. Bioscience, Biotechnology and Biochemistry 65:126-132. [ Links ] Kumar, K.; Chaudhary, L. C.; Agarwal, N. and Kamra, D. N. 2014. Effect of feeding tannin degrading bacterial culture (Streptococcus gallolyticus strain TDGB 406) on nutrient utilization, urinary purine derivatives and growth performance of goats fed on Quercus semicarpifolia leaves. Journal of Animal Physiology and Animal Nutrition 98:879-885. [ Links ] Lavin, S. R.; Chen, Z. and Abrams, S. A. 2010. Effect of tannic acid on iron absorption in straw-colored fruit bats (Eidolon helvum). Zoo Biology 29:335-343. [ Links ] Lavin, S. R. 2012. Plant phenolics and their potential role in mitigating iron overload disorder in wild animals. Journal of Zoo Wildlife Medicine 43:S74-S82. [ Links ] Le Bourvellec, C. and Renard, C. M. G. C. 2012. Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Critical Reviews in Food Science and Nutrition 52:213-248. [ Links ] Leathwick, D. M. and Besier, R. B. 2014. The management of anthelmintic resistance in grazing ruminants in Australasia-strategies and experiences. Veterinary Parasitology 204:44-54. [ Links ] Lee, S. H.; Shinde, P. L.; Choi, J. Y.; Kwon, I. K.; Lee, J. K.; Pak, S. I.; Cho, W. T. and Chae, B. J. 2010. Effects of tannic acid supplementation on growth performance, blood hematology, iron status and faecal microflora in weanling pigs. Livestock Science 131:281-286. [ Links ] Li, Z. P.; Liu, H. L.; Li, G. Y.; Bao, K.; Wang, K. Y.; Xu, C.; Yang, Y. F.; Yang, F. H. and Wright, A.-D. G. 2013. Molecular diversity of rumen bacterial communities from tannin-rich and fiber-rich forage fed domestic Sika deer (Cervus nippon) in China. BMC Microbiology 13:1-12. [ Links ] Lorenz, M. M.; Alkhafadji, L.; Stringano, E.; Nilsson, S.; Mueller- Harvey, I. and Udén, P. 2014. Relationship between condensed tannin structures and their ability to precipitate feed proteins in the rumen. Journal of the Science of Food and Agriculture 94:963-968. [ Links ] Lou, H.; Yamazaki, Y.; Sasaki, T.; Uchida, M.; Tanaka, H. and Oka, S. 1999. A-type proanthocyanidins from peanut skins. Phytochemistry 51:297-308. [ Links ] Mahmood, S.; Ali, H.; Ahmad, F. and Iqbal, Z. 2014. Estimation of tannins in different sorghum varieties and their effects on nutrient digestibility and absorption of some minerals in caged white leghorn layers. International Journal of Agriculture and Biology 16:217-221. [ Links ] Maitland, P.; Nierenstein, M.; Mitchell, C. A.; Lynch, G. R.; Norman, P. J.; Hughes, E. B. and Bagnall, H. H. 1936. Tea and coffee, with special reference to their alkaloids and tannins. Analyst 61:288-314. [ Links ] Mangino, J.; Peterson, K. and Jacobs, H. 2003. Development of an emissions model to estimate methane from enteric fermentation in cattle. U. S. Environ. Prot. Agency. Available at: <http://www.epa.gov/ttnchie1/conference/ei12/green/mangino.pdf>. Accessed on: Jan. 14, 2016. [ Links ] McArthur, C.; Sanson, G. and Beal, A. M. 1995. Salivary proline-rich proteins in mammals: roles in oral homeostasis and counteracting dietary tannin. Journal of Chemical Ecology 21:663-691. [ Links ] McDonald, M.; Mila, I. and Scalbert, A. 1996. Precipitation of metal ions by plant polyphenols: optimal conditions and origin of precipitation. Journal of Agriculture and Food Chemistry 44:599-606. [ Links ] McNabb, W. C.; Waghorn, G. C.; Peters, J. S. and Barry, T. N. 1996. The effect of condensed tannin in Lotus pedunculatus on the solubilization and degradation of ribulose-1,5-bisphosphate carboxylase protein in the rumen and the sites of Rubisco digestion. British Journal of Nutrition 76:535-549. [ Links ] McNabb, W. C.; Peters, J. S.; Foo, L. Y.; Waghorn, G. C. and Jackson, F. S. 1998. Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1). Journal of the Science of Food and Agriculture 77:201-212. [ Links ] McSweeney, C. S.; Collins, E. M. C.; Blackall, L. L. and Seawright, A. A. 2008. A review of anti-nutritive factors limiting potential use of Acacia angustissima as a ruminant feed. Animal Feed Science Technology 147:158-171. [ Links ] Merchen, N. R. and Titgemeyer, E. C. 1992. Manipulation of amino acid supply to the growing ruminant. Journal of Animal Science 70:3238-3247. [ Links ] Min, B. R.; Barry, T. N.; Attwood, G. T. and McNabb, W. C. 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Animal Feed Science and Technology 106:3-19. [ Links ] Min, B. R.; Pinchak, W. E.; Fulford, J. D. and Puchala, R. 2005. Wheat pasture bloat dynamics, in vitro ruminal gas production, and potential bloat mitigation with condensed tannins. Journal of Animal Science 83:1322-1331. [ Links ] Min, B. R.; Solaiman, S.; Terrill, T.; Ramsay, A. and Mueller-Harvey, I. 2015. The effects of tannins-containing ground pine bark diet upon nutrient digestion, nitrogen balance, and mineral retention in meat goats. Journal of Animal Science and Biotechnology 6:25. [ Links ] Minho, A. P.; Bueno, I. C. S.; Louvandini, H.; Jackson, F.; Gennari, S. M. and Abdalla, A. L. 2008. Effect of Acacia molissima tannin extract on the control of gastrointestinal parasites in sheep. Animal Feed Science and Technology 147:172-181. [ Links ] Mladénka, P.; Macáková, K.; Filipsky, T.; Zatloukalová, L.; Jahodár, L.; Bovicelli, P.; Silvestri, I. P.; Hrdina, R. and Saso, L. 2011. In vitro analysis of iron chelating activity of flavonoids. Journal of Inorganic Biochemistry 105:693-701. [ Links ] Mole, S.; Butler, L. G. and Iason, G. 1990. Defense against dietary tannin in herbivores: A survey for proline rich salivary proteins in mammals. Biochemical Systematics and Ecology 18:287-293. [ Links ] Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C. and Bartolomé, B. 2010. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food & Function 1:233-253. [ Links ] Mueller-Harvey, I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture 86:2010-2037. [ Links ] Nam, J.-W.; Phansalkar, R. S.; Lankin, D. C.; McAlpine, J. B.; Leme-Kraus, A. A.; Vidal, C. M. P.; Gan, L.-S.; Bedran-Russo, A.; Chen, S.-N. and Pauli, G. F. 2017. Absolute configuration of native oligomeric proanthocyanidins with dentin biomodification potency. Journal of Organic Chemistry 82:1316-1329. [ Links ] Naumann, H. D.; Muir, J. P.; Lambert, B. D.; Tedeschi, L. O. and Kothmann, M. M. 2013a. Condensed tannins in the ruminant environment: A perspective on biological activity. Journal of Agricultural Sciences 1:8-20. [ Links ] Naumann, H. D.; Tedeschi, L. O.; Muir, J. P.; Lambert, B. D. and Kothmann, M. M. 2013b. Effect of molecular weight of condensed tannins from warm-season perennial legumes on ruminal methane production in vitro. Biochemical Systematics and Ecology 50:154-162. [ Links ] Naumann, H. D.; Tedeschi, L. O.; Hagerman, A. E.; Lambert B. D. and Muir, J. P. 2013c. Methane emission and protein precipitating ability of condensed tannins from warm-season perennial legumes. p.491-492. In: Energy and protein metabolism and nutrition in sustainable animal production, EAAP publication No. 134. Oltjen, J. W.; Kebreab, E. and Lapierre, H., eds. Wageningen Academic Publishers. Wageningen, The Netherlands. [ Links ] Naumann, H. D.; Armstrong, S. A.; Lambert, B. D.; Muir, J. P.; Tedeschi, L. O. and Kothmann, M. M. 2014a. Effect of molecular weight and concentration of legume condensed tannins on in vitro larval migration inhibition of Haemonchus contortus. Veterinary Parasitology 199:93-98. [ Links ] Naumann, H. D.; Hagerman, A. E.; Lambert, B. D.; Muir, J. P.; Tedeschi, L. O. and Kothmann, M. M. 2014b. Molecular weight and protein-precipitating ability of condensed tannins from warm-season perennial legumes. Journal of Plant Interactions 9:212-219. [ Links ] Naumann, H. D.; Cherry, N. M.; Tedeschi, L. O.; Muir, J. P. and Lambert, B. D. 2014c. A conceptual model of protein-precipitable polyphenols (condensed tannins) on protein binding and protein digestions in ruminants. Journal of Animal Science 92(E-Suppl. 2):910. [ Links ] Naumann, H. D.; Fonseca, M. A. and Tedeschi, L. O. 2015a. Predicting ruminal methane inhibition by condensed tannins using nonlinear exponential decay regression analysis. Journal of Animal Science 93:5341-5345. [ Links ] Naumann, H. D.; Lambert, B. D.; Armstrong, S. A.; Fonseca, M. A.; Tedeschi, L. O.; Muir, J. P. and Ellersieck, M. R. 2015b. Effect of replacing alfalfa with panicled-tick clover or sericea lespedeza in corn-alfalfa-based substrates on in vitro ruminal methane production. Journal of Dairy Science 98:3980-3987. [ Links ] Ng, F.; Kittelmann, S.; Patchett, M. L.; Attwood, G. T.; Janssen, P. H.; Rakonjac, J. and Gagic, D. 2016. An adhesin from hydrogenutilizing rumen methanogen Methanobrevibacter ruminantium M1 binds a broad range of hydrogen-producing microorganisms. Environmental Microbiology 18:3010-3021. doi: 10.1111/1462-2920.13155. [ Links ] O’Donovan, L. and Brooker, J. D. 2001. Effect of hydrolysable and condensed tannins on growth, morphology and metabolism of Streptococcus gallolyticus (S. caprinus) and Streptococcus bovis. Microbiology 147:1025-1033. [ Links ] Odenyo, A. A.; Osuji, P. O.; Karanfil, O. and Adinew, K. 1997. Microbiological evaluation of Acacia angustissima as a protein supplement for sheep. Animal Feed Science and Technology 65:99-112. [ Links ] Orlandi, T.; Kozloski, G. V.; Alves, T. P.; Mesquita, F. R. and Ávila, S. C. 2015. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Animal Feed Science and Technology 210:37-45. [ Links ] Ozdal, T.; Capanoglu, E. and Altay, F. 2013. A review on protein- phenolic interactions and associated changes. Food Research International 51:954-970. [ Links ] Patra, A. K. and Saxena, J. 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. Journal of the Science of Food and Agriculture 91:24-37. [ Links ] Perez-Gregorio, M. R.; Mateus, N. and De Freitas, V. 2014. Rapid screening and identification of new soluble tannin-salivary protein aggregates in saliva by mass spectrometry (MALDI-TOF-TOF and FIA-ESI-MS). Langmuir 30:8528-8537. [ Links ] Perron, N. R. and Brumaghim, J. L. 2009. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochemistry and Biophysics 53:75-100. [ Links ] Poncet-Legrand, C.; Edelmann, A.; Putaux, J.-L.; Cartalade, D.; Sarni-Manchado, P. and Vernhet, A. 2006. Poly(-proline) interactions with flavan-3-ols units: influence of the molecular structure and the polyphenol/protein ratio. Food Hydrocolloids 20:687-697. [ Links ] Powell, J. M.; Aguerre, M. J. and Wattiaux, M. A. 2010. Tannin extracts abate ammonia emissions from simulated dairy barn floors. Journal of Environmental Quality 40:907-914. [ Links ] Quideau, S.; Deffieux, D.; Douat-Casassus, C. and Pouységu, L. 2011. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie International Edition 50:586-621. [ Links ] Quijada, J.; Fryganas, C.; Ropiak, H. M.; Ramsay, A.; Mueller-Harvey, I. and Hoste, H. 2015. Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. Journal of Agriculture and Food Chemistry 63:6346-6354. [ Links ] Ramin, M. and Huhtanen, P. 2013. Development of equations for predicting methane emissions from ruminants. Journal of Dairy Science 96:2476-2493. [ Links ] Redondo, L. M.; Chacana, P. A.; Dominguez, J. E. and Fernandez Miyakawa, M. E. 2014. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Fronteirs in Microbiology 5:1-7. [ Links ] Roche, J. F. 2006. The effect of nutritional management of the dairy cow on reproductive efficiency. Animal Reprodion Science 96:282-296. [ Links ] Saminathan, M.; Tan, H. Y.; Sieo, C. C.; Abdullah, N.; Wong, C. M. V. L.; Abdulmalek, E. and Ho, Y. W. 2014. Polymerization degrees, molecular weights and protein-binding affinities of condensed tannin fractions from a leucaena leucocephala hybrid. Molecules 19:7990-8010. [ Links ] Santos-Buelga, C. and Scalbert, A. 2000. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. Journal of the Science of Food and Agriculture 80:1094-1117. [ Links ] Scalbert, A.; Morand, C.; Manach, C. and Rémésy, C. 2002. Absorption and metabolism of polyphenols in the gut and impact on health. Biomedicine and Pharmacotherapy 56:276-282. [ Links ] Schwab, C. G. 1995. Protected proteins and amino acids for ruminants. p.115-141. In: Biotechnology in animal feeds and animal feeding. Wallace, R. J. and Chesson, A., eds. VCH, New York, NY. [ Links ] Sell, D. R.; Reed, W. M.; Chrisman, C. L. and Rogler, J. C. 1984. Mucin excretion and morphology of the intestinal tract as influenced by sorghum tannins. Nutrition Reports International 31:1369-1374. [ Links ] Shaik, S. A.; Terrill, T. H.; Miller, J. E.; Kouakou, B.; Kannan, G.; Kaplan, R. M.; Burke, J. M. and Mosjidis, J. A. 2006. Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Veterinary Parasitology 139:150-157. [ Links ] Smith, A. H.; Zoetendal, E. and Mackie, R. I. 2005. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microbial Ecology 50:197-205. [ Links ] Soares, S.; Vitorino, R.; Osório, H.; Fernandes, A.; Venâncio, A.; Mateus, N.; Amado, F. and De Freitas, V. 2011. Reactivity of human salivary proteins families toward food polyphenols. Journal of Agriculture and Food Chemistry 59:5535-5547. [ Links ] Soares, S.; Mateus, N. and De Freitas, V. 2012a. Carbohydrates inhibit salivary proteins precipitation by condensed tannins. Journal of Agriculture and Food Chemistry 60:3966-3972. [ Links ] Soares, S.; Sousa, A.; Mateus, N. and De Freitas, V. 2012b. Effect of condensed tannins addition on the astringency of red wines. Chemical Senses 37:191-198. [ Links ] Spencer, C. M.; Cai, Y.; Martin, R.; Gaffney, S. H.; Goulding, P. N.; Magnolato, D.; Lilley, T. H. and Haslam, E. 1988. Polyphenol complexation-some thoughts and observations. Phytochemistry 27:2397-2409. [ Links ] Tamilmani, P. and Pandey, M. C. 2016. Iron binding efficiency of polyphenols: Comparison of effect of ascorbic acid and ethylen ediaminetetraacetic acid on catechol and galloyl groups. Food Chemistry 197:1275-1279. [ Links ] Tedeschi, L. O.; Callaway, T. R.; Muir, J. P. and Anderson, R. C. 2011. Potential environmental benefits of feed additives and other strategies for ruminant production. Revista Brasileira de Zootecnia 40:291-309. [ Links ] Tedeschi, L. O.; Ramírez-Restrepo, C. A. and Muir, J. P. 2014. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 8:1095-1105. [ Links ] Terrill, T. H.; Mosjidis, J. A.; Moore, D. A.; Shaik, S. A.; Miller, J. E.; Burke, J. M.; Muir, J. P. and Wolfe, R. 2007. Effect of pelleting on efficacy of sericea lespedeza hay as a natural dewormer in goats. pdf. Veterinary Parasitology 146:117-122. [ Links ] Terrill, T.; Dykes, G.; Shaik, S.; Miller, J.; Kouakou, B.; Kannan, G.; Burke, J. and Mosjidis, J. 2009. Efficacy of sericea lespedeza hay as a natural dewormer in goats: Dose titration study. Veterinary Parasitology 163:52-56. [ Links ] Theodoridou, K.; Aufrère, J.; Andueza, D.; Pourrat, J.; Le Morvan, A.; Stringano, E.; Mueller-Harvey, I. and Baumont, R. 2010. Effects of condensed tannins in fresh sainfoin (Onobrychis viciifolia) on in vivo and in situ digestion in sheep. Animal Feed Science Technology 160:23-38. [ Links ] Tian, Y.; Zou, B.; Li, C.-M.; Yang, J. Xu, S.-F. and Hagerman, A. E. 2012. High molecular weight persimmon tannin is a potent antioxidant both ex vivo and in vivo. Food Research International 45:26-30. [ Links ] Tshuma, T.; Holm, D. E.; Fosgate, G. T. and Lourens, D. C. 2014. Pre-breeding blood urea nitrogen concentration and reproductive performance of Bonsmara heifers within different management systems. Tropical Animal Health and Production 46:1023-1030. [ Links ] USEPA. 2016. U.S. Greenhouse Gas Inventory Report: 1990-2014. Available at: <https://www3.epa.gov/climatechange/ghgemissions/usinventoryreport.html>. Accessed on: July 1, 2016. [ Links ] Van Duinkerken, G.; Andre, G.; Smits, M. C. and Monteny, G. J. and Sebek, L. B. 2005. Effect of rumen-degradable protein balance and forage type on bulk milk urea concentration and emission of ammonia. Journal of Dairy Science 88:1099-1112. [ Links ] Vargas-Magana, J. J.; Aguilar-Caballero, A. J.; Torres-Acosta, J. F. J.; Sandoval-Castro, C. A.; Hoste, H. and Capetillo-Leal, C. M. 2013. Tropical tannin-rich fodder intake modifies saliva-binding capacity in growing sheep. Animal 7:1921-1924. [ Links ] Waghorn, G. C.; Ulyatt, M. J.; John, A. and Fisher, M. T. 1987. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. British Journal of Nutrition 57:115-126. [ Links ] Waghorn, G. C.; Shelton, I. D. and McNabb, W. C. 1994a. Effect of condensed tannins in Lotus pedunculatas on its nutritive value for sheep. 1. Non-nitrogenous aspects. Journal of Agricultural Science 123:99-107. [ Links ] Waghorn, G. C.; Shelton, I. D.; McNabb, W. C. and McCutcheon, S. N. 1994b. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogenous aspects. Journal of Agricultural Science 123:109-119. [ Links ] Waghorn, G. 2008. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production— Progress and challenges. Animal Feed Science and Technology 147:116-139. [ Links ] Westwood, C. T.; Lean, I. J.; Garvin, J. K. and Wynn, P. C. 2000. Effects of genetic merit and varying dietary protein degradability on lactating dairy cows. Journal of Dairy Science 83:2926-2940. [ Links ] Williams, C. M.; Eun, J.-S.; Dschaak, C. M.; MacAdam, J. W.; Min, B. R. and Young, A. J. 2010. CASE STUDY: In vitro ruminal fermentation characteristics of birdsfoot trefoil (Lotus corniculatus L.) hay in continuous cultures. The Professional Animal Scientist 26:570-576. [ Links ] Williams, C. M.; Eun, J.-S.; MacAdam, J. W.; Young, A. J.; Fellner, V. and Min, B. R. 2011. Effects of forage legumes containing condensed tannins on methane and ammonia production in continuous cultures of mixed ruminal microorganisms. Animal Feed Science and Technology 166-167:364-372. [ Links ] Wischer, G.; Greiling, A. M.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K. and Rodehutscord, M. 2014. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 8:938-948. [ Links ] Wood, C.; Fang, S. G.; Hunt, A.; Streich, W. J. and Clauss, M. 2003. Increased iron absorption in lemurs: quantitative screening and assessment of dietary prevention. American Journal of Primatology 61:101-110. [ Links ] Wren, A. F.; Cleary, M.; Frantz, C.; Melton, S. and Norris, L. 2013. 90-Day oral toxicity study of a grape seed extract (IH636) in rats. Journal of Agriculture and Food Chemistry 50:2180-2192. [ Links ] Yun, S.; Habicht, J.-P.; Miller, D. D. and Glahn, R. P. 2004. An in vitro digestion/Caco-2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. Journal of Nutrition 134:2717-2721. [ Links ] Received: June 08, 2017; Accepted: September 05, 2017 *Corresponding author:naumannhd@missouri.edu This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.