
Archives of Animal Nutrition
Vol. 64, No. 2, April 2010, 121-135
Effect of tannins on growth performance and intestinal ecosystem in
weaned piglets
Giacomo Biagia*, Irene Cipollinia, Brigitte R. Paulicksb and Franz X. Rothb
aDipartimento di Morfofisiologia Veterinaria e Produzioni Animali (DIMORFIPA), Universita`
di Bologna, Ozzano Emilia, Italy;bDivision of Animal Nutrition and Production Physiology,
Technical University of Munich, Freising-Weihenstephan, Germany
(Received 23 May 2009; accepted 2 October 2009)
Tannins are natural polyphenolic compounds that can reduce digestibility of
dietary protein but also display antibacterial effects. The present study
investigated, in vitro and in vivo, the effect of different levels of tannins (using a
chestnut wood extract containing
75% tannins) on growth performance,
intestinal microbiota and wall morphology in piglets. During a 24 h in vitro
caecal fermentation, the utilisation of tannins at 0.75, 1.5, 3, and 6 g/l significantly
reduced total gas production and concentrations of ammonia and volatile fatty
acids and increased viable counts of enterococci and coliforms. When fed to
piglets at 1.13, 2.25, and 4.5 g/kg, tannins significantly improved feed efficiency
and reduced caecal concentrations of ammonia, iso-butyric, and iso-valeric acid.
Viable counts of lactobacilli tended to be increased by tannins in the jejunum,
while bacterial caecal counts were not affected. Depth of ileal crypts tended to
decrease in piglets fed tannins at 2.25 and 4.5 g/kg. The present study showed that
feeding weaned piglets with a tannin-rich wood extract can result in improved
feed efficiency and reduction of intestinal bacterial proteolytic reactions. The
growth-enhancing effect that tannins had on enterococci and coliforms under
in vitro conditions deserves further investigation.
Keywords: caecum; microbiota; performance; pigs; tannins
1. Introduction
Tannins are natural polyphenolic compounds that are found in many vegetable
feedstuffs and can be extracted from the wood of several trees
(Kumar and
Vaithiyanathan 1990). Tannins have been known for the detrimental effect that they
exert on animal growth performance when tannin-rich feedstuffs are fed to non-
ruminants. In fact, tannins can reduce digestibility of dietary protein (Mariscal-
Landın et al. 2004) due to their ability to form insoluble complexes with both dietary
protein (Butler et al. 1984) and digestive enzymes (Jansman et al. 1994). Besides their
antinutritional properties, tannins also display beneficial antibacterial (Ahn et al.
1998; Min et al. 2007) and antidiarrhoic (Palombo 2006) effects. In the first weeks
after weaning, piglets frequently experience diarrhoea and low weight gain due to the
relative immaturity of their gastrointestinal and immune system (Hedemann and
Jensen
2004). Because bacteria-caused diarrhoea is the consequence of the
*Corresponding author. Email: giacomo.biagi@unibo.it
ISSN 1745-039X print/ISSN 1477-2817 online
2010 Taylor & Francis
DOI: 10.1080/17450390903461584

122
G. Biagi et al.
multiplication of harmful bacteria in the piglet intestine, this problem was
counteracted with antibiotics and auxinic agents that may as a side-effect select
antibiotic-resistant genes in the intestinal flora with the possibility of a transfer to
human pathogens (Phillips et al. 2004). Because of these concerns, the use of
antibiotic growth promoters has been prohibited within the European Union since
January 2006, thus increasing the need for non-pharmacological alternatives to
modulate the intestinal microbiota of young animals. Dietary supplementation with
tannins might reduce the incidence of diarrhoea in piglets but the effect of extracted
tannins on animal growth performance raises some concerns. The objective of the
present study was to investigate the effect of different levels of tannins on growth
response and metabolism of swine caecal microbiota in vitro and the effect of feeding
tannins on piglet growth performance, intestinal microbiota and wall morphology
in vivo.
2. Materials and methods
2.1. Animal care and use
Animal housing and care were conducted under supervision of the Veterinarian
Office of the Bavarian Government. The handling protocol ensured proper care and
treatment of all animals in conformity with the German law for animal protection.
2.2. In vitro study
The in vitro study was conducted at the Department of Veterinary Morphophysiol-
ogy and Animal Production (DIMORFIPA), University of Bologna, Italy, following
the procedure as described in Biagi et al. (2006).
A diet (57% corn, 16% barley, and 14% soybean meal) for pigs was pre-digested
in vitro to simulate the ileal digestion as described by Vervaeke et al. (1989). This is a
two-step procedure where feed is first incubated in a pepsin-HCl solution and then in
a pancreatin solution. The pre-digested diet was used as substrate in the in vitro
fermentation study (Biagi et al. 2006). The caecal content of six pigs (10 months old,
live weight 160 kg, fed a commercial corn-barley-soybean based diet) was collected
after slaughtering, diluted with buffer (ratio 1:2; McDougall 1948) and filtered. The
filtered liquid was used as inoculum. The inoculum was dispensed into five 10 ml
glass syringes (5 ml of inoculum in each syringe) and five 50 ml vessels (25 ml of
inoculum in each vessel) per treatment, containing 20 and 100 mg of pre-digested
diet
(used as control diet), respectively. Syringes and vessels were sealed and
incubated at 398C for 24 h.
There were five treatments: the control diet, and the control diet plus tannins
(Farmatan1, Tanin Sevnica, Slovenia) at 0.75, 1.5, 3.0, and 6.0 g/l. In all tannin
treatments, tannins were added at incubation start, prior to sealing syringes and
vessels. The pH of the inoculum was adjusted to 6.7.
Farmatan is a chestnut (Castanea sativa mill) wood extract (obtained by water
extraction) containing 75% tannins, mainly gallotannins (European Food Safety
Authority [EFSA] 2005). Gallotannins are hydrolysable tannins that are formed
from gallic acid (and ellagic acid) which is linked to OH groups of sugars (especially
glucose). Other components of Farmatan include water (6.3%), ellagic acid (13.2%),
gallic acid (1.7%), simple sugars (2.6%), crude protein (0.8%), mineral substances
(1.0%), and crude fibre (0.2%).
Archives of Animal Nutrition
123
Gas production was measured as described by Menke et al. (1979), using 10 ml
glass syringes and recording the cumulative volume of gas produced every 30 min.
Samples of fermentation fluid were collected from each vessel at 6 and 24 h of
incubation for pH and ammonia and at 24 h for volatile fatty acids (VFA) and
viable counts of bacteria determinations.
2.3. In vivo feeding study
The in vivo feeding trial was conducted at the Division of Animal Nutrition and
Production Physiology, Technical University of Munich, Germany. Forty-eight
crossbred piglets
(German Landrace 6 Pietrain) were weaned at
28 d and
transported from the piggery to the barn where they were housed in individual
cages in a controlled environment for a 28 d trial period. After a 4 d adaptation
period during which all piglets received the same base diet, animals (8.23 + 0.93 kg
BW) were divided into four groups (12 animals per group), homogenous for
weight, gender and litter. Pigs received the base diet with: (i) no addition (control
diet), or with the addition of tannins (Farmatan1) at (ii) 1.13 g/kg, (iii) 2.25 g/kg,
and (iv)
4.5 g/kg. All diets were formulated to provide the same amount of
energy, protein, essential amino acids, calcium and phosphorus. No antibacterial
agents were added to diets. Feed and water were provided on an ad libitum
basis. Composition and chemical analyses of the experimental diets are reported in
Table 1.
Animals were individually weighed and feed consumption was recorded for each
pig weekly. The amount of feed wasted was recorded daily. Animal health was
monitored throughout the trial.
On day 28, six animals per treatment were killed by electrical stunning followed
by complete bleeding. Within 20 min after death, intestinal content (whole content
of jejunum and caecum and the content of the last 100 cm of the ileum were
Table 1. Composition of the basal diet used in the in vivo feeding trial.*
Ingredient
Content [g/kg]
Chemical composition
Content
Corn meal
305
Dry matter{ [g/kg]
885
Wheat meal
273
Crude protein{ [g/kg]
190
Barley meal
171
Fat{ [g/kg]
33.9
Soybean meal, 47% CP
161
Crude fibre{ [g/kg]
32.0
Soybean oil
10
Ca [g/kg]
8.5
Potato protein
40
P [g/kg]
6.0
CaCO3
5.7
Lysine [g/kg]
12.0
Ca(H2PO4)2
5.7
Methionine [g/kg]
3.9
Premix#
20
Methionine þ cysteine [g/kg]
7.2
L-lysine HCl
6.6
Threonine [g/kg]
7.8
DL-methionine
1.0
Tryptophan [g/kg]
2.4
L-threonine
1.0
ME [MJ/kg]
13.5
Trypthophan
0.2
Notes: *As-fed; experimental diets were obtained replacing 0, 1.5, 3, and 6 g corn meal per kg diet by an
equal amount of Farmatan1 (containing 75% tannins).#Provided per kg of diet: Ca, 6.16 g; P, 1.68 g;
Na, 1.40 g; Mg, 0.28 g; Vitamin A, 16,800 IU; Vitamin D3, 1,680 IU; Vitamin E, 56 mg; Vitamin K3, 1 mg;
Thiamine, 1 mg; Riboflavin, 3.5 mg; Pyridoxine, 2.1 mg; Vitamin B12, 0.03 mg; Nicotinic acid, 16.8 mg;
d-Pantothenic acid,
8.4 mg; Folic acid,
2.8 mg; Biotin,
0.22 mg; Choline,
420 mg; Fe,
140 mg;
Zn, 140 mg; Cu, 28 mg; Mn, 56 mg; I, 1.68 mg; Se, 0.36 mg.{Determined by analysis.

124
G. Biagi et al.
separately collected) and mucosa (samples were obtained 150 cm distal from the
pylorus, 100 cm before the ileo-caecal valve, and at the apical portion of the caecum
for jejunum, ileum, and caecum, respectively) were sampled for pH and ammonia
determination and for intestinal mucosa morphology analysis, while VFA were
determined only in caecal samples. Samples from the jejunum and the caecum were
also cultured for viable counts of bacteria.
2.4. Chemical analyses of feed, faecal samples, fermentation fluid and intestinal
contents
Analyses of the diets (CP, crude fibre, and ether extract) were performed according
to the Association of Official Analytical Chemists standard methods (AOAC 2000;
Method 954.01 for CP, Method 962.09 for crude fibre, and Method 920.39 for ether
extract).
Ammonia in fermentation fluid and intestinal chyme was measured according to
Searcy et al. (1967) using a commercial kit (Urea/BUN – Color, BioSystems SA,
Barcelona, Spain). For the determination of VFA in the intestinal chyme, the digesta
were diluted 1:2 with distilled water and centrifuged (14,000 g, 10 min) and 1 ml of
the supernatant was filled in microfuge tubes and deproteinised with 50 ml perchloric
acid (Merck, Darmstadt, Germany). After 3 h the samples were centrifuged again
(14,000 g, 10 min). For the determination of VFA in fermentation fluid, samples
were centrifuged
(3,000 g,
15 min). Concentration of VFA in supernatants of
fermentation fluid and digesta was determined by gas chromatography (Biagi et al.
2006).
2.5. Bacterial counts
Immediately after collection of the chyme samples, 1 g sample was diluted with 9 ml
of a 1% peptone solution and homogenised. Viable counts of bacteria in chyme
(n ¼ 6) and fermentation fluid (n ¼ 5) samples were measured by plating serial 10-
fold dilutions (in 1% peptone solution) onto Lactobacillus Medium III agar plates
(Medium 638, DSMZ, Germany) for lactobacilli, Difco DRCM agar plates
(Beckton, Dickinson and Company, Franklin Lakes, NJ, USA) for clostridia, Azide
Maltose agar plates (Biolife, Milano, Italy) for enterococci, and MacConkey agar
plates
(N.
1.05465, Merck, Darmstadt, Germany) for coliforms. Lactobacillus
Medium III and DRCM agar plates were incubated for 48 h at 398C under
anaerobic conditions
(H2 þ approximately 4 to 10% CO2; BBL GasPak Plus
Anaerobic System Envelopes, Beckton, Dickinson and Company, Sparks, MD,
USA). Azide Maltose agar and MacConkey agar plates were incubated for 24 h at
398C under aerobic conditions.
2.6. Morphological evaluations
The height of villi and depth of crypts on mucosa samples from jejunum, ileum and
caecum were assessed as described in Biagi et al. (2006). Mucosa samples were fixed
in 10% buffered Carson’s formalin and embedded in paraffin; histological sections of
3 mm were obtained from tissue blocks, cut perpendicular to the mucosa surface and
stained with haematoxylin and eosin. Histomorphometric measurements were
performed using a computer-assisted image-analysis system
(Cytometrica, Byk

Archives of Animal Nutrition
125
Gulden, Milan, Italy) to assess height of 10 villi (except for caecum, in which villi are
absent) and depth of 10 crypts of each section, on random-selected microscopic
fields.
2.7. Statistical analyses
2.7.1. In vitro fermentation
A modified Gompertz bacterial growth model (Zwietering et al. 1992) was used to fit
gas production data. This model assumes that substrate levels limit growth in a
logarithmic relationship (Schofield et al. 1994) as follows:
V ¼ VF expf exp½1 þ ðmme=VFÞðl tÞ g;
where V ¼ volume of gas produced at time t, t ¼ fermentation time, VF ¼
maximum volume of gas produced, mm ¼ maximum rate of gas production, which
occurs at the point of inflection of the gas curve, and l ¼ the lag time, as the time-
axis intercept of a tangent line at the point of inflection (Zwietering et al. 1990).
Curve fitting was performed using the program GraphPad Prism 4.0 (GraphPad
Software, San Diego, CA, USA). Data were analysed by ANOVA using the GLM
procedure of SAS (SAS Inst., Inc., Cary, NC, USA) in a completely randomised
design. Linear and quadratic contrasts were used to determine the nature of the
response exhibited to the addition of tannins. Each syringe and vessel formed the
experimental unit. Differences were considered statistically significant at p 5 0.05.
2.7.2. In vivo feeding trial
Data were analysed by ANOVA using the GLM procedure of SAS (SAS Inst., Inc.,
Cary, NC, USA) in a completely randomised design. Linear and quadratic contrasts
were used to determine the nature of the response exhibited to the feeding of tannins.
Each piglet formed the experimental unit. Differences were considered statistically
significant at p 5 0.05.
3. Results
3.1. In vitro experiment
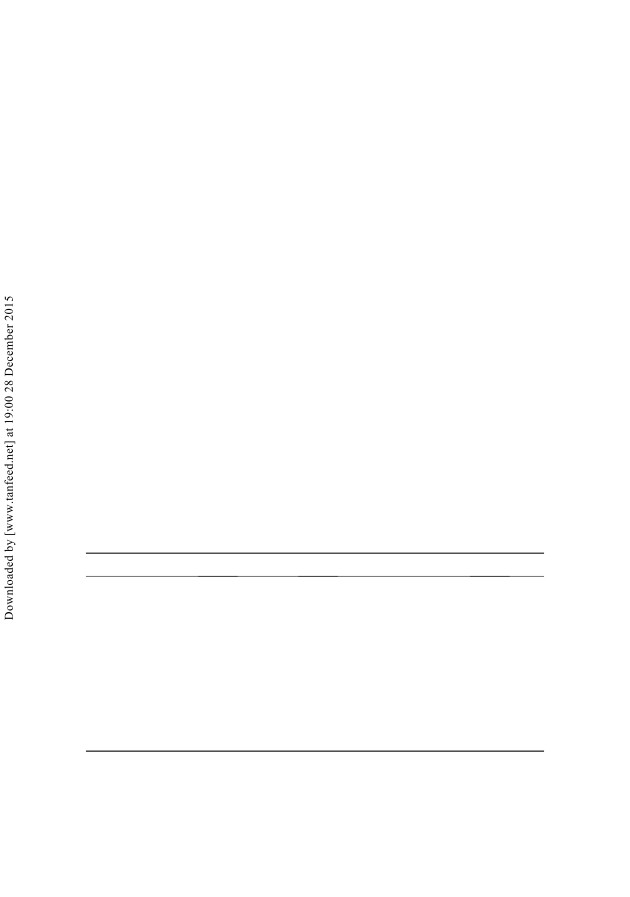
Gas production curves
(Figure
1) were accurately described by the modified
Gompertz model (r2 ¼ 0.92). Gas production results are shown in Table 2. Total gas
production was reduced by tannins (linear, p 5 0.05), whereas maximum rate of gas
production tended to be increased in tannin containing vessels (p ¼ 0.06).
After 6 h of incubation, pH values were significantly reduced by tannins (linear,
p 5 0.001; Table 2), but tannin supplementation had no effect on pH at 24 h.
Ammonia concentrations were significantly reduced by tannins after 6 h (linear
and quadratic, p 5 0.001; Table 3) and this reduction ranged from 730% when
tannins were added at 0.75 g/l to 740% with tannins used at 6 g/l. After 24 h of
incubation, ammonia was significantly reduced by tannins (linear and quadratic,
p 5 0.001) from 732% to 763% for tannins at 0.75 g/l and 6 g/l, respectively.
After 24 h of incubation, compared with the control, acetic acid was reduced by
tannins
(linear and quadratic, p 5 0.01; Table
3) and the lowest acetic acid
126
G. Biagi et al.
Figure 1. Gas production curves from in vitro incubation of swine caecal inoculum with or
without addition of different concentrations of tannins. Gas production was monitored with
five syringes per treatment individually fitted to the modified Gompertz growth model. Means
are plotted and bars indicate SEM.
Table 2. Modified Gompertz equation fitted to gas production data and pH from the 24 h
in vitro incubation of swine caecal inoculum with different concentrations of tannins.
Added tannins [g/kg]
Contrasts, p
Pooled
p of the
0
0.75
1.5
3
6
SEM model
Linear Quadratic
VF*
4.37
4.25
4.24
2.91
3.86
0.264
0.011
0.031
0.487
mm#
0.29
0.30
0.31
0.31
0.48
0.037
0.056
0.013
0.069
pH at 6 h
7.03
6.99
6.94
6.96
6.87
0.017
50.001
50.001
0.865
pH at 24 h
6.80
6.80
6.82
6.86
6.81
0.030
0.711
0.472
0.679
Notes: *VF, Maximum volume of gas produced [ml];#mm, Maximum rate of gas production [ml/h]. Values
are least squares means of five replicates for each diet tested.
concentration was determined at 6 g tannins per litre (716%). Propionic acid (linear
and quadratic, p 5 0.05) and n-butyric acid (linear and quadratic, p 5 0.01) were
significantly reduced by tannins from 4-12% and from 8-36% for tannins at 0.75
and 6 g/l, respectively. Concentrations of iso-butyric and iso-valeric acid were
significantly (linear and quadratic, p 5 0.001) reduced by tannins and this reduction
ranged from 21-52% and from 29-57% for tannins at 0.75 and 6 g/l, respectively.
Total concentration of volatile fatty acids was significantly reduced by tannins
(linear, p 5 0.001).
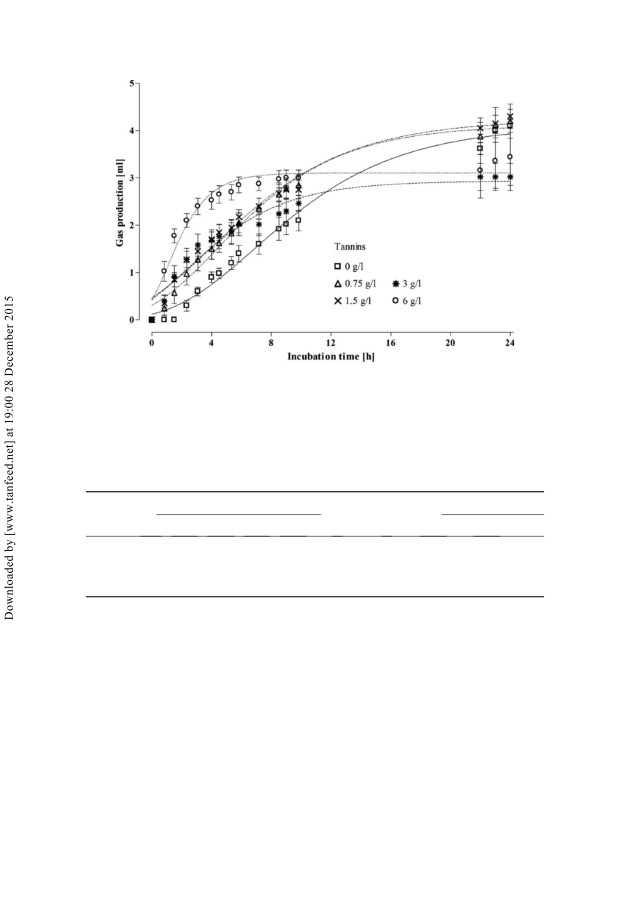
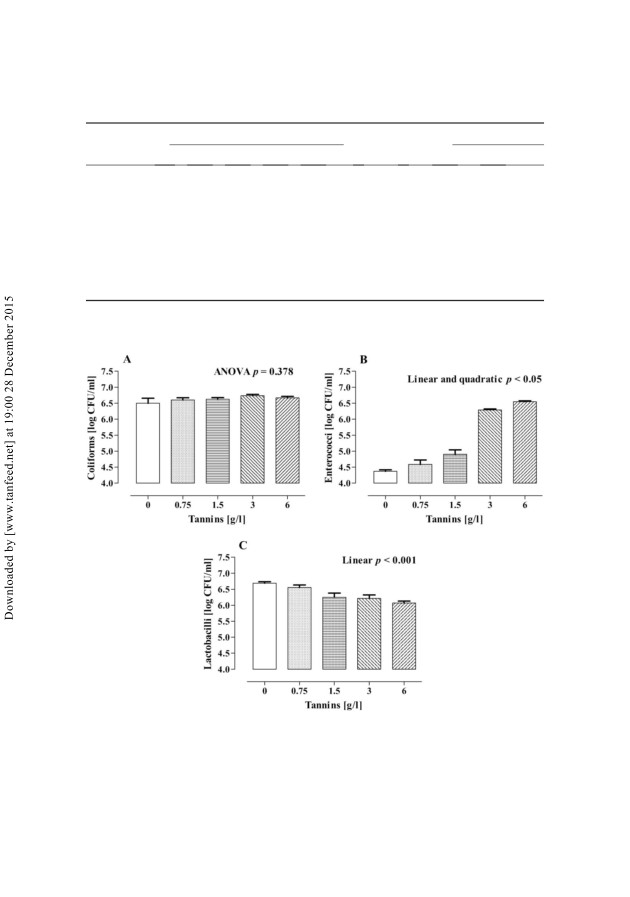
Viable counts of bacteria at 6 and 24 h are represented in Figures 2 and 3,
respectively. At 6 h, viable counts of coliforms were not affected by treatment;
conversely, after 24 h of incubation, coliforms were increased (linear and quadratic,
Archives of Animal Nutrition
127
Table 3. Concentrations [mmol/l] of ammonia (after 6 and 24 h) and volatile fatty acids
(after 24 h) from an in vitro incubation of swine caecal inoculum with different concentrations
of tannins.
Added tannins [g/kg]
Contrasts, p
Pooled
p of the
0
0.75
1.5
3
6
SEM model
Linear
Quadratic
Ammonia
at 6 h
12.4
8.66
8.12
7.97
7.37
0.232
50.001
50.001
50.001
at 24 h
20.6
14.1
9.50
8.91
7.72
0.435
50.001
50.001
50.001
Acetic acid
42.8
44.2
41.8
38.0
36.1
0.511
50.001
50.001
50.01
Propionic acid
16.9
16.3
15.4
13.9
15.0
0.267
50.001
50.001
50.05
iso-Butyric acid
1.22
0.96
0.84
0.67
0.58
0.009
50.001
50.001
50.001
n-Butyric acid
9.10
8.39
7.71
7.28
5.81
0.088
50.001
50.001
50.01
iso-Valeric acid
1.03
0.73
0.63
0.50
0.45
0.013
50.001
50.001
50.001
C2/C3*
2.53
2.71
2.72
2.74
2.41
0.004
50.001
0.159
50.001
n-C4/isoC4#
7.47
8.72
9.17
10.8
9.95
0.010
50.001
50.001
50.001
Total acids
72.4
71.7
67.5
61.4
58.9
0.792
50.001
50.001
0.117
Notes: *C2/C3, Acetic to propionic acid ratio;#n-C4/isoC4, n-Butyric to iso-butyric acid ratio. Values are
least squares means of five replicates for each diet tested.
Figure 2. Viable counts of coliforms (A), enterococci (B), and lactobacilli (C) at 6 h of an
in vitro incubation of pig caecal inoculum with different concentrations of tannins (values are
means + SEM of five vessels per treatment).
p 5 0.01) by tannins with the highest concentration of coliforms in vessels added
with tannins at 3 g/l (þ0.5 log CFU/ml). Lactobacilli were significantly reduced by
tannin addition and the lowest counts of lactobacilli were determined at 6 g tannins
128
G. Biagi et al.
Figure 3. Viable counts of coliforms (A), enterococci (B), and lactobacilli (C) at 24 h of an
in vitro incubation of pig caecal inoculum with different concentrations of tannins (Values are
means + SEM of five vessels per treatment).
per litre (70.7 and 70.8 log CFU/ml after 6 and 24 h of incubation, respectively;
linear, p 5 0.01). Enterococci counts were significantly increased by tannins, with
the highest counts of tannins at 6 g/l (þ2.2 and þ2.5 log CFU/ml after 6 and 24 h of
incubation, respectively; linear and quadratic, p 5 0.05). Viable counts of clostridia
were not affected by treatment and averaged 7.7 and 7.8 log CFU/ml after 6 and 24 h
of incubation, respectively (data not shown).
3.2. In vivo feeding trial
Animal live weight, average daily gain, and daily feed intake were not significantly
influenced by tannins
(Table 4). Feed efficiency throughout the
28 d trial was
significantly improved by tannins (linear, p 5 0.05) and the lowest feed efficiency
was observed in pigs fed tannins at 4.5 g/kg (75%; Table 4).
Experimental diets had no significant effect on intestinal pH; average pH values
were 5.70, 6.53, and 5.77 in jejunum, ileum, and caecum, respectively (data not
shown). Caecal ammonia concentrations showed a tendency towards a reduction in
pigs fed tannins (p ¼ 0.07; Table 5), while no effect was observed on ammonia levels
in chyme samples from jejunum and ileum.
Concentration of propionic acid in the caecal chyme showed a tendency towards
a reduction in tannin-fed pigs (p ¼ 0.06; Table 5). Feeding tannins to piglets reduced

Archives of Animal Nutrition
129
Table 4. Growth performances of piglets receiving a diet added or not with different levels of
tannins in the four weeks after weaning.
Added tannins [g/kg]
Contrasts, p
Pooled
p of the
0
1.13
2.25
4.5
SEM model
Linear Quadratic
Final BW [kg]
19.3
19.4
19.7
20.7
0.680
0.478
0.152
0.549
ADG [g/d]
395
400
410
445
17.30
0.183
0.045
0.388
ADFI [g/d]
549
553
570
588
23.79
0.638
0.214
0.752
Feed efficiency
1.39
1.38
1.39
1.32
0.019
0.034
0.023
0.105
Note: Values are least squares means of 12 piglets for each diet tested.
Table 5. Concentrations [mmol/l] of ammonia in chyme from jejunum, ileum and caecum
and volatile fatty acids in chyme from caecum from piglets that had received a diet added or
not with different concentrations of tannins in the four weeks after weaning.
Added tannins [g/kg]
Contrasts, p
Pooled
p of the
0
1.13
2.25
4.5
SEM model
Linear Quadratic
Ammonia
Jejunum
12.6
14.2
12.8
12.3
1.201
0.721
0.725
0.420
Ileum
20.2
16.7
17.7
16.5
1.788
0.497
0.270
0.553
Caecum
37.6
27.5
30.6
25.4
3.022
0.071
0.039
0.449
Acetic acid
74.1
61.5
75.2
65.6
4.695
0.151
0.590
0.305
Propionic acid
43.4
35.2
45.8
36.0
3.009
0.058
0.428
0.809
iso-Butyric acid
1.56
0.88
1.01
0.71
0.141
0.003
0.002
0.212
n-Butyric acid*
17.5
13.7
20.8
14.0
1.385
0.006
0.613
0.314
iso-Valeric acid
1.53
0.87
0.93
0.72
0.124
0.001
50.001
0.095
Total acids*
142
116
148
119
7.526
0.016
0.298
0.892
Notes: Values are least squares means of six piglets for each diet tested. *Cubic (p 5 0.01) when
comparing 0 through 4.5 g/kg of tannins.
(linear, p 5 0.01) caecal concentrations of iso-butyric (from 735% to 755% for
tannins at 2.25 and 4.5 g/kg, respectively) and iso-valeric acid (from 739% to
753% for tannins at 2.25 and 4.5 g/kg, respectively). Dietary treatments also had a
significant effect on n-butyric and total VFA concentrations (cubic, p 5 0.01).
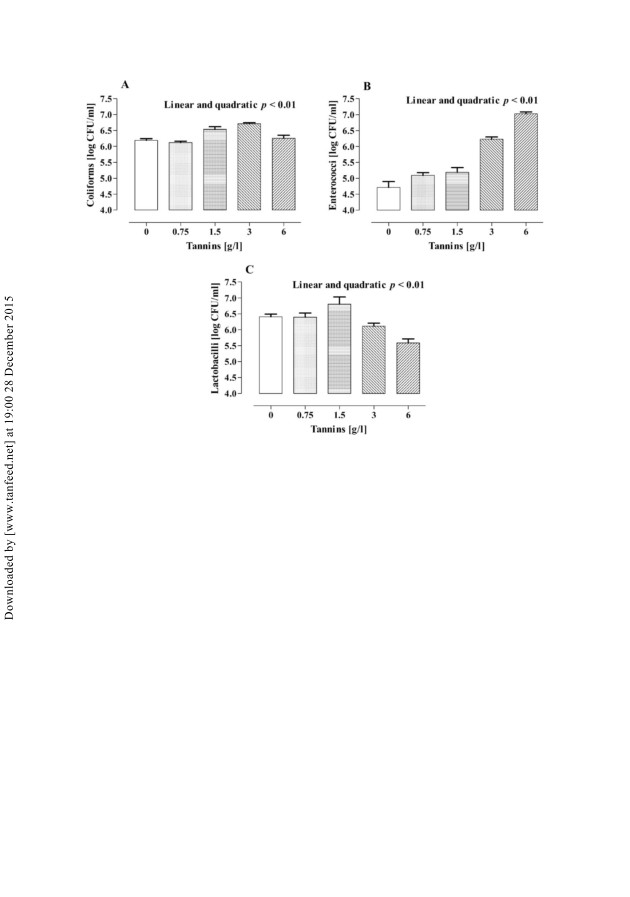
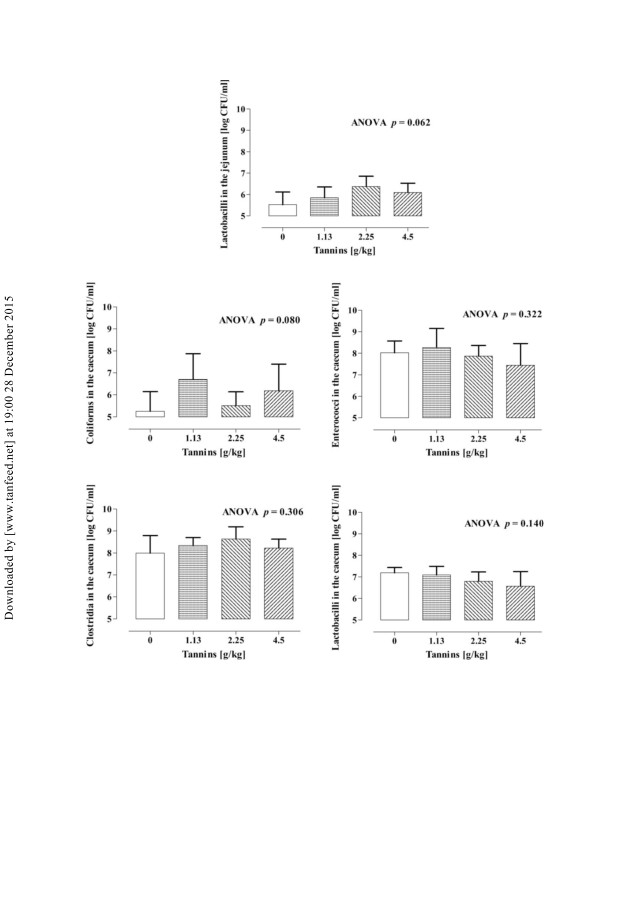
Viable counts of bacteria in jejunum and caecum chyme are represented in
Figure 4. Feeding tannins tended (p ¼ 0.06) to increase viable counts of lactobacilli
in the jejunum, while caecal lactobacilli were not influenced by dietary treatment.
Coliforms, enterococci and clostridia concentrations in the jejunum were under the
detection limit and could not be determined. Caecal counts of coliforms showed a
tendency towards an increase when pigs were fed tannins (p ¼ 0.08), whereas caecal
clostridia and enterococci were not affected by tannin supplementation. Tannin
supplementation had no effect on villi length in the jejunum and ileum (Table 6) but
significantly influenced ileal crypt depth (cubic, p 5 0.05).
4. Discussion
In the present trial, the utilisation of tannins did not significantly influence animal
weight gain but improved feed efficiency when tannins were fed at 4.5 g/kg of diet.
130
G. Biagi et al.
Figure 4. Viable counts of coliforms, enterococci, clostridia, and lactobacilli in the jejuna
and caecal chyme of piglets fed different concentrations of tannins (values are means + SEM
of six animals per treatment).
The improved utilisation of the diet in piglets fed high levels of tannins is a
controversial finding because tannins have been known as antinutritional factors for
their ability to reduce digestibility of dietary protein (Mariscal-Landın et al. 2004). In
a study by Lizardo et al. (1995), the addition to pig diets of a tannin-rich variety of
sorghum resulted in reduced animal growth performance. Conversely, other authors
observed that feeding pigs with feedstuffs high in tannins such as field beans (Flis

Archives of Animal Nutrition
131
Table 6. Villous height and crypt depth [mm] of the intestinal mucosa in piglets that had
received a diet added or not with different levels of tannins in the four weeks after weaning.
Added tannins [g/kg]
Contrasts, p
Pooled
p of the
0
1.13
2.25
4.5
SEM
model
Linear
Quadratic
Jejunum
Villi
413
466
442
466
26.78
0.457
0.272
0.589
Crypts
356
396
358
360
31.01
0.768
0.859
0.557
Ileum
Villi
313
328
297
314
27.11
0.881
0.806
0.969
Crypts*
272
310
226
241
19.74
0.033
0.059
0.587
Caecum
Crypts
348
425
348
358
25.22
0.120
0.666
0.197
Notes: Values are least squares means of six piglets for each diet tested. *Cubic (p 5 0.05) when
comparing 0 through 4.5 g/kg of tannins.
et al. 1999) and carob powder (Lizardo et al. 2002) did not affect animal growth. In a
study with weaned pigs (Myrie et al. 2008), feeding animals with tannins at 15 g/kg
of diet did not affect animal growth performance despite the fact that the ileal
apparent digestibility was reduced for threonine but not for total protein. Longstaff
and McNab (1991) observed that tannin-rich fieldbeans at low doses enhanced the
activity of lipase in digesta from both the jejunum and ileum of young chicks. When
reduced growth performance is reported in monogastric animals fed sorghum
(Lizardo et al. 1995), faba beans (Rubio et al. 1990), lupins (Kim et al. 2007) as well
as other tannins-rich feedstuffs, it has to be considered that, when compared to more
traditional feedstuffs for pigs such as soybean meal and corn meal, these feedstuffs
are generally characterised by lower digestibility of their starch
(Rooney and
Pflugfelder 1986; Wiseman 2006) and protein (Mariscal-Landın et al. 2002) fractions,
and by the presence of significant amounts of other antinutritional factors (Huisman
et al. 1990) that altogether might result in reduced animal growth.
In the present in vitro study, tannins had a strong influence on the metabolism of
swine caecal microbiota. In fact, increasing concentrations of tannins linearly
reduced total gas production and concentrations of acetic, propionic, and n-butyric
acid, showing an inhibitory effect on the activity of caecal bacteria. Because the
acetic to propionic acid ratio was not linearly influenced by tannin addition, it seems
that tannins reduced total bacterial activity without favouring specific metabolic
pathways. Conversely, increasing concentrations of tannins resulted in a linear
increase of the n-butyric to iso-butyric acid ratio. This finding might be explained by
a relatively higher carbohydrate metabolism of butyric acid producing bacteria,
together with a reduction of iso-butyric acid, a metabolite deriving from protein
bacterial catabolism.
Interestingly, tannins at 4.5 g/kg determined the highest rate of gas production,
suggesting that, despite a general inhibitory effect, the activity of some bacteria was
selectively stimulated by high levels of tannins. In fact, counts of enterococci (and, to
a lower extent, of coliforms) were significantly increased by tannins, whereas
lactobacilli counts were reduced. When fed to piglets, tannins did not increase
enterococci caecal counts but tended to increase lactobacilli in the jejunum and
coliforms in the caecum. It has been shown that several intestinal bacterial strains

132
G. Biagi et al.
can degrade tannic, gallic, and ellagic acids and use them as a source of energy (Bhat
et al. 1998; Cerda et al. 2005). Despite the relatively low content of simple sugars
(2.6%) of the wood extract, it is also possible that the extract might contain some
growth factors for enterococci that exerted their action in vitro but could not reach
the animal hindgut when tannins were fed to piglets, as such failing to increase
enterococci caecal counts. In vitro, the enhanced growth of enterococci might explain
the slight reduction of lactobacilli counts due to higher competition for substrates.
Nevertheless, higher enterococci counts might simply be the consequence of higher
growth potential of enterococci compared to lactobacilli.
The definition of tannins refers to a group of water-soluble polyphenols that are
classified as hydrolysable and non-hydrolysable (condensed) tannins (Akiyama et al.
2001). Tannin compounds differ in their antibacterial activity (Akiyama et al. 2001;
Puupponen-Pimia et al. 2005) and the composition of tannins is influenced by plant
of origin and method of extraction. In vitro, antibacterial effects of tannins against
pathogenic strains of Escherichia coli (Yao et al. 2006), Clostridium perfringens and
Staphylococcus aureus (Ahn et al. 1998), and Helicobacter pylori (Funatogawa et al.
2004) have been reported, but in our study tannins at any concentration tested did
not reduce in vitro counts of coliforms and clostridia. In the study by Ahn et al.
(1998), tannins were obtained from wattle and showed antibacterial activity against
different bacterial strains with MIC values ranging from 0.5-8 mg/ml that were
comparable to the tannin concentrations that we used in the present trial.
The lack of in vitro antibacterial activity against coliforms and clostridia of the
tannins that we used was confirmed by the in vivo study. At present, there are no data
regarding the amount of dietary tannins that are absorbed in the small intestine of
pigs, so that it is difficult to estimate how many tannins will reach the animal
hindgut. Studies in rats fed tannins have indicated that tannic acid was mostly
recovered in the faeces, but small amounts of tannin compounds were found in
plasma and urine samples, showing that absorption of tannins occurred (Clifford
and Brown 2005). At present, there is no evidence that tannins might exert in the
animal intestine the same effective antibacterial effect that has been observed by
some authors (Ahn et al. 1998; Funatogawa et al. 2004; Yao et al. 2006) under
in vitro conditions.
Concentrations of ammonia, iso-butyric acid, and iso-valeric acid were
significantly reduced both in vivo and in vitro, showing that tannins effectively
reduced bacterial proteolytic reactions. In fact, the above isoacids are formed from
the deamination of valine and leucine (Van Soest 1982) and are indicative as
ammonia of bacterial protein catabolism extent. Ammonia is a toxic compound that
can destroy cells and reduce villus height (Nousiainen 1991), and once absorbed
must be converted to urea with the loss of energy (Eisemann and Nienaber 1990).
Moreover, subacute concentrations of ammonia may reduce animal performance
(Visek 1978). Several dietary supplements have been shown to reduce intestinal
bacterial proteolysis: among these are non-digestible oligosaccharides and probiotics
(Piva et al. 2005), organic acids (Roth and Kirchgessner 1998), and herb extracts
(Ushida et al. 2002). Based on the present results, tannins seem to be a valuable
alternative to the aforementioned dietary supplements in order to control intestinal
bacterial proteolytic reactions in weaned pigs.
The effect of tannin supplementation on intestinal mucosa histology was also
investigated. Early weaning has a dramatic negative impact on the intestinal mucosa
morphology of piglets (Gu et al. 2002) and can lead to nutrient malabsorption

Archives of Animal Nutrition
133
(Boudry et al. 2004). n-Butyric acid is the preferred energy substrate of the mucosa of
the ileum (Chapman et al. 1995) and large intestine (Roediger 1980), and it has been
observed that feeding piglets with sodium butyrate (Galfi and Bokori 1990; Wang
et al. 2005) can have a positive trophic effect on the intestinal mucosa, increasing the
length of ileal microvilli and the depth of caecal crypts. Conversely, other authors
failed to observe any trophic effect on the intestinal mucosa when sodium butyrate
(Biagi et al. 2007) or butyric acid precursors (Piva et al. 2002) were fed to weaned
pigs. In the present study, tannins had a cubic effect on n-butyric acid caecal
concentrations and the depth of ileal crypts tended to decrease when tannins were
used at 2.25 and 4.5 g/kg. At present, we do not have an explanation for this effect of
tannins. Nevertheless, because crypts in the small intestine mainly have a secretory
function (Wood 2006), a reduction of their surface might help reducing the severity
of piglet post-weaning diarrhoea.
5. Conclusion
The present study has produced evidence that feeding weaned piglets with a tannin-
rich wood extract can result in improved feed efficiency and reduction of intestinal
bacterial proteolytic reactions. Nevertheless, the growth-enhancing effect that the
wood extract seemed to have on coliforms deserves further investigation.
References
Ahn YJ, Lee CO, Kweon JH, Ahn JW, Park JH. 1998. Growth-inhibitory effects of Galla
Rhois-derived tannins on intestinal bacteria. J Appl Microbiol. 84:439-443.
Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. 2001. Antibacterial action of several
tannins against Staphylococcus aureus. J Antimicrob Chemother. 48:487-491.
Association of Official Analytical Chemists (AOAC). 2000. Official methods of analysis. 17th
ed. Gaithersburg (MD): W Horwitz.
Bhat TK, Singh B, Sharma OP. 1998. Microbial degradation of tannins
– a current
perspective. Biodegradation. 9:343-357.
Biagi G, Piva A, Moschini M, Vezzali E, Roth FX. 2006. Effect of gluconic acid on piglet
growth performance, intestinal microflora, and intestinal wall morphology. J Anim Sci.
84:370-378.
Biagi G, Piva A, Moschini M, Vezzali E, Roth FX. 2007. Performance, intestinal microflora,
and wall morphology of weanling pigs fed sodium butyrate. J Anim Sci. 85:1184-1191.
Boudry G, Peron V, Huerou-Luron I, Lalles JP, Seve B. 2004. Weaning induces both transient
and long-lasting modifications of absorptive, secretory, and barrier properties of piglet
intestine. J Nutr. 134:2256-2262.
Butler L, Riedl DJ, Lebryk DG, Blytt HJ. 1984. Interactions of proteins with sorghum
tannins: Mechanism, specificity and significance. J Assoc Off Chem Soc. 61:916-920.
Cerda B, Periago P, Espın JC, Tomas-Barberan FA. 2005. Identification of urolithin a as a
metabolite produced by human colon microflora from ellagic acid and related compounds.
J Agric Food Chem. 53:5571-5576.
Chapman MA, Grahn MF, Hutton M, Williams NS. 1995. Butyrate metabolism in the
terminal ileal mucosa of patients with ulcerative colitis. Brit J Surg. 82:36-38.
Clifford MN, Brown JE.
2005. Flavonoids: Chemistry, biochemistry and applications.
Chapter 6: Dietary flavonoids and health – broadening the perspective. Boca Raton (FL):
CRC Press/Taylor & Francis. p. 331-370.
Eisemann JH, Nienaber JA. 1990. Tissue and whole-body oxygen uptake in fed and fasted
steers. Brit J Nutr. 64:399-411.
EFSA (European Food Safety Authority). 2005. Opinion of the scientific panel on additives
and products or substances used in animal feed on a request from the commission on the
safety and efficacy of the product Farmatan for rabbits and piglets. EFSA J. 222:1-20.

134
G. Biagi et al.
Flis M, Sobotka W, Purwin C, Zdunczyk Z. 1999. Nutritional value of diets containing field
bean (Vicia faba L.) seeds with high or low proanthocyanidin levels for pig. J Anim Feed
Sci. 8:171-180.
Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y. 2004.
Antibacterial activity of hydrolyzable tannins derived from medicinal plants against
Helicobacter pylori. Microbiol Immunol. 48:251-261.
Galfi P, Bokori J. 1990. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta
Vet Hung. 38:3-17.
Gu X, Li D, She R. 2002. Effect of weaning on small intestinal structure and function in the
piglet. Arch Anim Nutr. 56:275-286.
Hedemann MS, Jensen BB. 2004. Variations in enzyme activity in stomach and pancreatic
tissue and digesta in piglets around weaning. Arch Anim Nutr. 58:47-59.
Huisman J, van der Poel AFB, Verstegen MWA, van Weerden EJ. 1990. Antinutritional
factors (ANF) in pig production. World Rev Anim Prod. 25:77-82.
Jansman AJM, Enting H, Verstegen MWA, Huisman J. 1994. Effects of condensed tannins in
hulls of faba beans (Vicia faba L.) on the activity of trypsin and chymotrypsin in digesta
collected from the small intestine of pigs. Brit J Nutr. 71:627-641.
Kim JC, Pluske JR, Mullan BP. 2007. Lupins as a protein source in pig diets. CAB Rev
Perspect Agric Vet Sci Nutr Nat Resource. 2(No. 003):12.
Kumar R, Vaithiyanathan S. 1990. Occurrence, nutritional significance and effect on animal
productivity of tannins in tree leaves. Anim Feed Sci Technol. 30:21-38.
Lizardo R, Canellas J, Mas F, Torrallardona D, Brufau J. 2002. Utilisation of carob powder
in piglet diets and its influence on growth performance and health after weaning. 34emes
Journees de la Recherche Porcine, sous l’egide de l’Association Francaise de Zootechnie.
Paris, France. p. 5-7. Fevrier 2002.
Lizardo R, Peiniau J, Aumaitre A. 1995. Effect of sorghum on performance, digestibility of
dietary components and activities of pancreatic and intestinal enzymes in the weaned
piglet. Anim Feed Sci Technol. 56:67-82.
Longstaff MA, McNab JM. 1991. The effect of concentration of tannin-rich bean hulls (Vicia
faba L.) on activities of lipase (EC 3.1.1.3) and alpha-amylase (EC 3.2.1.1) in digesta
and pancreas and on the digestion of lipid and starch by young chicks. Brit J Nutr.
66:139-147.
Mariscal-Landın G, Avellaneda JH, Reis de Souza TC, Aguilera A, Borbolla GA, Mar B.
2004. Effect of tannins in sorghum on amino acid ileal digestibility and on trypsin and
chymotrypsin activity of growing pigs. Anim Feed Sci Technol. 117:245-264.
Mariscal-Landın G, Lebreton Y, Seve B.
2002. Apparent and standardised true ileal
digestibility of protein and amino acids from faba bean, lupin and pea, provided as whole
seeds, dehulled or extruded in pig diets. Anim Feed Sci Technol. 97:183-198.
McDougall EI. 1948. Studies on ruminant saliva. 1. The composition and output of sheep’s
saliva. Biochem J. 43:99-109.
Menke KH, Raab L, Salewski A, Steingass H, Fritz H, Schneider W. 1979. The estimation of
digestibility and metabolizable energy content of ruminant feeding stuffs from the gas
production when they are incubated with rumen liquor in vitro. J Agric Sci. 93:217-222.
Min ER, Pinchak WE, Anderson RC, Callaway TR. 2007. Effect of tannins on the in vitro
growth of Escherichia coli O157:H7 and in vivo growth of generic Escherichia coli excreted
from steers. J Food Prot. 70:543-550.
Myrie SB, Bertolo RF, Sauer WC, Ball RO. 2008. Effect of common antinutritive factors and
fibrous feedstuffs in pig diets on amino acid digestibilities with special emphasis on
threonine. J Anim Sci. 86:609-619.
Nousiainen J. 1991. Comparative observations on selected probiotics and olaquindox as feed
additives for piglets around weaning. 2. Effect on villus length and crypt depth in the
jejunum, ileum, caecum and colon. J Anim Physiol Anim Nutr. 66:224-230.
Palombo EA. 2006. Phytochemicals from traditional medicinal plants used in the treatment
of diarrhoea: Modes of action and effects on intestinal function. Phytother Res.
20:717-724.
Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, Nightingale C, Preston R,
Waddell J. 2004. Does the use of antibiotics in food animals pose a risk to human health?
A critical review of published data. J Antimicrob Chemother. 53:28-52.

Archives of Animal Nutrition
135
Piva A, Luchansky JB, Biagi G. 2005. Effect of lactitol, lactic acid bacteria, or their com-
binations (synbiotic) on intestinal proteolysis in vitro, and on feed efficiency in weaned
pigs. Can J Anim Sci. 85:345-353.
Piva A, Prandini A, Fiorentini L, Morlacchini M, Galvano F, Luchansky JB. 2002. Tributyrin
and lactitol synergistically enhanced the trophic status of the intestinal mucosa and
reduced histamine levels in the gut of nursery pigs. J Anim Sci. 80:670-680.
Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. 2005. The action of
berry phenolics against human intestinal pathogens. Biofactors. 23:243-251.
Roediger WE. 1980. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa
in man. Gut. 21:793-798.
Rooney LW, Pflugfelder RL. 1986. Factors affecting starch digestibility with special emphasis
on sorghum and corn. J Anim Sci. 63:1607-1623.
Roth FX, Kirchgessner M. 1998. Organic acids as feed additives for young pigs: Nutritional
and gastrointestinal effects. J Anim Feed Sci. 7(Suppl1):25-33.
Rubio LA, Brenes A, Castano M. 1990. The utilization of raw and autoclaved faba beans
(Vicia faba L., var. minor) and faba bean fractions in diets for growing broiler chickens.
Brit J Nutr. 63:419-430.
Schofield P, Pitt RE, Pell AN. 1994. Kinetics of fiber digestion from in vitro gas production.
J Anim Sci. 72:2980-2991.
Searcy RL, Reardon JE, Foreman JA. 1967. A new photometric method for serum urea
nitrogen determination. Am J Med Technol. 33:15-20.
Ushida K, Maekawa M, Arakawa T. 2002. Influence of dietary supplementation of herb
extracts on volatile sulfur production in pig large intestine. J Nutr Sci Vitaminol. 48:18-23.
Van Soest PJ. 1982. Nutritional ecology of the ruminants. Corvallis (OR): O and B Books.
p. 162.
Vervaeke IJ, Dierick NA, Demeyer DL, Decuypere JA. 1989. Approach to the energetic
importance of fibre digestion in pigs. II. An experimental approach to hindgut digestion.
Anim Feed Sci Technol. 23:169-194.
Visek WJ. 1978. Diet and cell growth modulation by ammonia. Am J Clin Nutr. 31(Suppl. 10):
216-220.
Wang JF, Chen YX, Wang ZX, Dong SH, Lai ZW. 2005. Effect of sodium butyrate on the
structure of the small intestine mucous epithelium of weaning piglets. Chin J Vet Sci
Technol. 35:298-301.
Wiseman J. 2006. Variations in starch digestibility in non-ruminants. Anim Feed Sci Technol.
130:66-77.
Wood JD. 2006. Physiology of the gastrointestinal tract. Chapter
41: Pathophysio-
logy underlying the irritable bowel syndrome. Amsterdam: Elsevier Academic Press.
p.
1009-1032.
Yao K, He Q, Ying Jia D, Shi B. 2006. The potential of wattle tannin extracts for fine use. Nat
Prod Res. 20:271-278.
Zwietering MH, Jongenburger L, Rombouts FM, van’t Riet K. 1990. Modelling the bacterial
growth curve. Appl Environ Microbiol. 56:1875-1881.
Zwietering MH, Rombouts FM, van’t Riet K. 1992. Comparison of definitions of the lag
phase and the exponential phase in bacterial growth. J Appl Bacteriol. 72:139-145.