molecules
Review
The Effects of Tannins in Monogastric Animals with
Special Reference to Alternative Feed Ingredients
Zahra Mohammed Hassan
1
, Tlou Grace Manyelo
1,2
, Letlhogonolo Selaledi
1,3
and Monnye Mabelebele
1,
*
1
Department of Agriculture and Animal Health, College of Agriculture and Environmental Sciences,
University of South Africa, Florida 1710, South Africa; zahrabattal@gmail.com (Z.M.H.);
manyelo.t.g@gmail.com (T.G.M.); letlhogonolo.selaledi@up.ac.za (L.S.)
2
Department of Agricultural Economics and Animal Production, University of Limpopo,
Sovenga 0727, South Africa
3
Department of Zoology and Entomology, Mammal Research Institute, Faculty of Natural and Agricultural
Sciences, University of Pretoria, Hatfield 0028, South Africa
* Correspondence: mabelm@unisa.ac.za; Tel.: +27-11-471-3983
Academic Editors: Teresa Escribano-Bailón and Ignacio García-Estévez
Received: 16 September 2020; Accepted: 12 October 2020; Published: 14 October 2020
Abstract:
Over recent years, the monogastric animal industry has witnessed an increase in feed
prices due to several factors, and this trend is likely to continue. The hike in feed prices is mostly
due to extreme competition over commonly used conventional ingredients. For this trend to be
subdued, alternative ingredients of both plant and animal origin need to be sourced. These types of
ingredients are investigated with the aim of substituting all or some of the conventional compounds.
However, alternative ingredients often have a double-edged sword effect, in that they can supply
animals with the necessary nutrients although they contain antinutritional factors such as tannins.
Tannins are complex secondary metabolites commonly present in the plant kingdom, known to bind
with protein and make it unavailable; however, recently they have been proven to have the potential
to replace conventional ingredients, in addition to their health benefits, particularly the control of
zoonotic pathogens such as Salmonella. Thus, the purpose of this review is to (1) classify the types of
tannins present in alternative feed ingredients, and (2) outline the effects and benefits of tannins in
monogastric animals. Several processing methods have been reported to reduce tannins in diets for
monogastric animals; furthermore, these need to be cost-effective. It can thus be concluded that the
level of inclusion of tannins in diets will depend on the type of ingredient and the animal species.
Keywords:
antinutrients; feedstuffs; plant extracts; monogastric animals’ nutrition; tannins;
health benefits
1. Introduction
Monogastric animal production, in particular the poultry production sector, is growing
continuously, driven mostly by the demand for meat and eggs. However, this rapidly growing
industry and the increasing demand for poultry feeds have led to a considerable increase in feedstuff
prices. The gap between demand and supply of balanced feed is expected to increase, and consequently
increase the cost of production. On the other hand, the conventional feed ingredients such as maize,
wheat and rice can no longer meet the poultry industry’s demand for feed. In addition, in-feed
antibiotics have been used over a period of time as growth promoters, which positively aids in feed
conversion rates and consequently reduces the cost. However, it was discovered recently that the
inclusion of the antibiotics could leave residue in the meat and consequently cause resistance to
some bacteria in humans [
1
]. These multifaceted challenges compelled the concerned researchers
Molecules 2020, 25, 4680; doi:10.3390/molecules25204680 www.mdpi.com/journal/molecules
Molecules 2020, 25, 4680 2 of 17
to look for alternative ingredients which can fill the gap. Tannins are considered valid alternatives
to the conventional feed ingredients and as antipathogenic molecules, which can be used as an
alternative ingredient.
Tannins are a group of polyphenolic compounds commonly found in the plant kingdom [
2
].
Because they are antimicrobial, antiparasitic, antiviral, antioxidant, and anti-inflammatory [
2
], they are
considered valuable in that they can replace antibiotics in chicken feeds [
2
]. Although the use of
tannins in monogastric animals’ feed has been discouraged over the years because of the antinutrient
contents [
3
], recent studies have revealed that if tannins are used with caution, they can be of benefit to
monogastric animals [
4
]. Tannins also can decrease the risk of livestock diseases and the spread of
zoonotic pathogens. Current studies on the use of tannins in poultry production sector show favorable
outcomes [5].
The mechanism with which tannins promote growth in the monogastric animals are not as clear as
in ruminants [
2
]. The popular suggestion is that the inclusion of tannins in low concentrations leads to
an increase in feed intake and consequently the performance of monogastric animals [
2
]. There is also
a suggestion that the improvement in performance comes as a result of the creation of balance between
the negative effects of tannins on feed palatability and nutrient digestion and the positive effects on
promoting the health status of the intestinal ecology [
2
]. A study by [
6
] found that the condensed
tannins available in the extract of grape seed reduces the fecal shedding of E. Tenella, and an increased
growth performance of broiler chickens infected with E. Tenella.
To render tannins available to the monogastric animals, different processing methods to reduce
the antinutrient effects are recommended. For example, the reduction of the tannin component of
sorghum has improved its nutritional quality to become the closest alternative feed ingredient to maize
in poultry diets [
7
]. Lately, different processing methods were introduced to reduce the tannin content
in feed ingredients. The main methods used are cooking, dehulling, autoclaving, toasting, soaking,
using wood ash, adding tallow, and using tannin-binding agents and enzymes. Hence, the aims of
this review are(1) to elaborate on the use of tannins as alternative ingredient in monogastric animals’
feed; (2) to identify different structures and types of tannins; and (3) to identify successful processing
methods to reduce the harmful effects of tannins.
2. Methodology
This review was conducted according to the reporting items for systematic reviews and
meta-analyses (PRISMA) statement guidelines [
8
]. A comprehensive search was conducted to identify
eligible studies. Databases, namely Web of Science, Science Direct, Google Scholar, PubMed and Wiley
Online Database, were searched to obtain all relevant studies that were published before September
of 2020. The search strategy used involved a combination of the keywords “tannins”, “alternative
ingredients”, “monogastric animals”, “health benefits”, “condensed tannins”, “hydrolysable tannins”,
“medicinal uses of tannins”, “antinutrients in tannins”, “antibiotic resistance” and “tannin processing
methods”. Furthermore, the researchers narrowed their search to time scale 1977–2020 to include old
and new studies to draw a comparison between the uses of tannins in monogastric animals with the
current use. The search was not restricted by language, date, or study type. A total of 315 records were
screened after removal of duplicates. Later, 218 records were excluded because they were irrelevant.
The first draft articles were excluded for the following reasons: a) they did not cover the alternative
feed subject, b) some of the articles did not adequately address the importance of tannins in livestock
nutrition, c) some of the articles only focused on the undesirable antinutritional factors in the tannins.
A total of 97 records were initially used to prepare the review.
In the second stage, extra records were searched to include ‘’antibiotic resistant strains” to add to
the knowledge regarding antibiotic resistance and the health benefits of tannin. The overall number of
records used to prepare this review was 122 records.
Molecules 2020, 25, 4680 3 of 17
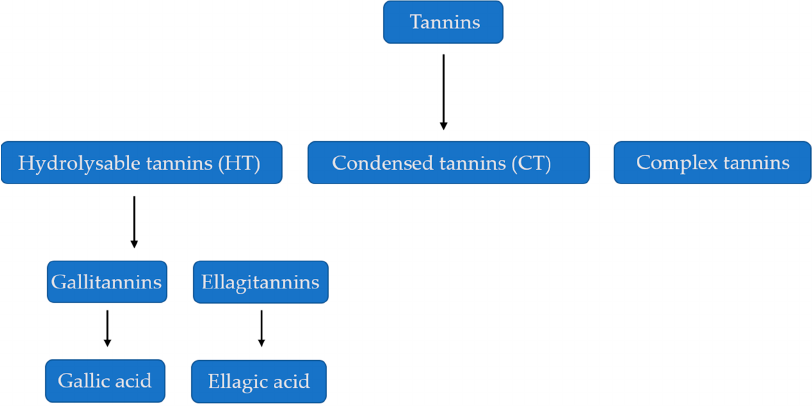
3. Structural Properties of Tannins
The physical and chemical properties of tannins differ according to the plant species [
9
]. Tannins are
classified into two main parts—the hydrolysable tannins (HTs) and condensed tannins (CTs), also known
as proanthocyanidins [
10
,
11
]. Hydrolysable tannins, as the name indicates, can be hydrolyzed by acids
or enzymes. Their structure is characterized by a polyol core [
12
]. On the other hand, the condensed
tannins are non-hydrolysable oligomeric and polymeric proanthocyanidins [
13
]. Condensed tannins
are where the coupling of the single units is by positioning of C-4 of the first unit with C-8 or C-6
of the second unit [
14
]. The two most common condensed tannins are the procyanidins and the
prodelphinidins [
12
]. There are three types of hydrolysable tannins, which include: gallotannins,
ellagitannins, and complex tannins and condensed tannins, called procyanidins [
15
], (Figure 1).
Gallic acid is mainly found in rhubarb and clove, while ellagic acid is found in eucalyptus leaves,
myrobalans and pomegranate bark [16].
Further to this, recent research showed that tannins are produced inside an organelle named
tannosome, which is believed to arise in cell plastids occurring in the green parts of plants that
contain chlorophyll pigments. After creation, the tannosome is encapsulated in a membrane, and later
transported to a plant vacuole for safe storage [
17
]. According to [
12
], the structures of the condensed
tannins from different species can be differentiated based on the proportion of trihydroxylated subunits,
ratio of cis/trans monomers, and the degree of polymerization. Figure 1 shows the classification of
tannins into different classes.
Molecules 2020, 25, x FOR PEER REVIEW 3 of 16
3. Structural Properties of Tannins
The physical and chemical properties of tannins differ according to the plant species [9]. Tannins
are classified into two main parts—the hydrolysable tannins (HTs) and condensed tannins (CTs), also
known as proanthocyanidins [10,11]. Hydrolysable tannins, as the name indicates, can be hydrolyzed
by acids or enzymes. Their structure is characterized by a polyol core [12]. On the other hand, the
condensed tannins are non-hydrolysable oligomeric and polymeric proanthocyanidins [13].
Condensed tannins are where the coupling of the single units is by positioning of C-4 of the first unit
with C-8 or C-6 of the second unit [14]. The two most common condensed tannins are the
procyanidins and the prodelphinidins [12]. There are three types of hydrolysable tannins, which
include: gallotannins, ellagitannins, and complex tannins and condensed tannins, called
procyanidins [15], (Figure 1). Gallic acid is mainly found in rhubarb and clove, while ellagic acid is
found in eucalyptus leaves, myrobalans and pomegranate bark [16].
Further to this, recent research showed that tannins are produced inside an organelle named
tannosome, which is believed to arise in cell plastids occurring in the green parts of plants that contain
chlorophyll pigments. After creation, the tannosome is encapsulated in a membrane, and later
transported to a plant vacuole for safe storage [17]. According to [12], the structures of the condensed
tannins from different species can be differentiated based on the proportion of trihydroxylated
subunits, ratio of cis/trans monomers, and the degree of polymerization. Figure 1 shows the
classification of tannins into different classes.
Figure 1. Classification of Tannins. Sources: [18,19].
4. Mode of Action and Functions of Tannins
Tannins are a complex group of polyphenolic compounds found in a wide range of plant species.
They are characterized by astringency and tanning properties, which are believed to be associated
with the higher molecular weight proanthocyanidins [20]. Hagerman [21] reported the molecular
weight of tannins to be between 500 and 5000 Da. They are found in wood, bark, leaves and fruits;
however, acacia species, which belong to the family of Leguminosae in the plant kingdom, are
considered the most common sources of tannins [22]. Previously, harmful nutritional consequences
have been attributed to tannins because they can precipitate proteins, inhibit digestive enzymes, and
decrease the utilization of vitamins and minerals [23]. In addition, it was assumed that tannins are
unabsorbable due to their high molecular weight and the ability to form insoluble structures with
components of food such as proteins [24]. Hagerman et al. [11] reported that tannins in poultry feed
affect dry matter intake and consequently the weight gain. Tannins that can be hydrolyzed are found
in smaller amounts in plants, while the condensed tannins are found in abundance. The concentration
Figure 1. Classification of Tannins. Sources: [18,19].
4. Mode of Action and Functions of Tannins
Tannins are a complex group of polyphenolic compounds found in a wide range of plant species.
They are characterized by astringency and tanning properties, which are believed to be associated with
the higher molecular weight proanthocyanidins [
20
]. Hagerman [
21
] reported the molecular weight of
tannins to be between 500 and 5000 Da. They are found in wood, bark, leaves and fruits; however,
acacia species, which belong to the family of Leguminosae in the plant kingdom, are considered
the most common sources of tannins [
22
]. Previously, harmful nutritional consequences have been
attributed to tannins because they can precipitate proteins, inhibit digestive enzymes, and decrease the
utilization of vitamins and minerals [
23
]. In addition, it was assumed that tannins are unabsorbable
due to their high molecular weight and the ability to form insoluble structures with components
of food such as proteins [
24
]. Hagerman et al. [
11
] reported that tannins in poultry feed affect dry
matter intake and consequently the weight gain. Tannins that can be hydrolyzed are found in smaller

Molecules 2020, 25, 4680 4 of 17
amounts in plants, while the condensed tannins are found in abundance. The concentration of tannins
is dependent on the plant genotype, tissue developmental stage, and the environmental conditions [
12
].
Biologically, tannins are significant in that they provide protection for the plant while still in the
plant and have potential effects after the plant has been harvested [
25
]. In recent research, tannins have
been proposed as an alternative to antibiotics because of the antimicrobial properties of tannins, which
is the ability to inhibit extracellular microbial enzymes. In addition, hydrolysable tannins could be used
in lieu of antibiotics, because bacteria such as Clostridium perfringens cannot develop resistance to them.
However, their use in animal feed is discouraged because they impact nutrition negatively. Their use
has been linked with lower feed intake and digestibility and leads to poorer animal performance.
Tannins have numerous applications that benefit humans. Some of the applications of tannins
include their use as nutraceuticals to prevent, for example, cancer, cardiovascular disease, kidney
disease, and diabetes [
26
]. They are also used for tanning leather, and manufacturing ink and
wood adhesives. Medicinally, tannins are homeostatic, antidiarrheal, and a remedy for alkaloid
and heavy-metals toxicity. In the lab, tannins are used as a reagent for protein detection, alkaloids,
and heavy metals due to their precipitating properties. In the food industry, tannins are used to clarify
wine, beer, and fruit juices. Other industrial uses of tannins include textile dyes, and as coagulants in
rubber production.
5. Antibiotic Resistance in Animal Byproducts
Antibiotic resistance is a concern for animal welfare and as a hazard to public health since the
contamination can be passed onto humans through the byproducts from animals. Although some
contributing factors are unavoidable, such as the ability of bacteria to adopt to the changing
environment [
27
], some of the factors are contributed by humans, such as the excessive use of
antibiotics for growth promotion in farm animals [
28
]. For example, antibiotic resistant salmonella
has been detected in meat [
29
]. Food animals are considered the main reservoir of antibiotic resistant
bacteria, which can be transferred to humans through zoonoses and the food chain [
30
,
31
]. Some of
the antibiotic resistant strains are presented in Table 1.
Table 1. Examples of antibiotic resistant strains in animal by-products.
Antibiotic Resistant Strains Animal Product References
Staphylococcus Cattle meat and milk [32]
Salmonella Poultry meat [33]
Campylobacter Poultry meat [34]
Escherichia coli
Cattle Liver and minced turkey meat
[35]
Escherichia coli Poultry meat [36]
Escherichia coli Poultry meat [37]
6. Medicinal Uses of Tannins
Tannins in plants are believed to function as chemical guards that protect the plants against
pathogens and herbivores, as stated by [
38
]. Furthermore, the properties of tannins as antioxidants
and reducing scavenging activities were also reported by [
39
]. The ability of tannins to chelate metals,
their antioxidant activity, antibacterial action, and complexation are believed to be the mechanism of
action behind tannins’ ability to treat and prevent certain conditions such as diarrhea and gastritis [
40
].
On the other hand, tannins’ mechanisms of antimicrobial activity include inhibition of extracellular
microbial enzymes, deprivation of the substrates required for microbial growth, or direct action on
microbial metabolism through inhibition of oxidative phosphorylation. The authors of [
41
] state that
the antimicrobial properties of tannins are believed to be associated with the hydrolysis of ester linkage
between gallic acid and polyols hydrolyzed after the ripening of many edible fruits, which enables the
Molecules 2020, 25, 4680 5 of 17
tannins to function as a natural defense mechanism against microbial infections. Table 2 demonstrates
some of the medicinal uses of tannins [42].
Table 2. Uses of tannins as medicinal sources and industrial agents.
Components Medicinal Uses References
Sweet chestnut extracts
Escherichia coli, Bacillus subtilis, Salmonella enterica serovar Enteritidis
[43]
Extract of chestnut shell
Enteritidis, Clostridium perfringens, Staphylococcus aureus, and
Campylobacter jejuni
[44]
Gall nuts Treatment of diarrhea and dermatitis [45]
Acacia Nilotica Antimutagenic and cytotoxic effects [46]
Sweet chestnut extracts Reduction of Salmonella infection [47]
Quebracho Tannins
Reduction of worm eggs counts and inhibition of development of
nematodes and lungworms
[48]
Chestnut extracts Control of Clostridium perfringens [49]
Pine needles and dry oak leaves Control of coccidian infection [50]
7. Tannins as Adhesives
Tannins are used as a partial or complete substitute for phenols in wood adhesives in the form of
tannin resin because of its phenolic structure [
51
]. The use of tannin adhesives was first successfully
traded in South Africa in early 1970s [
52
]. It is documented that previous research in the field of
fortified starch adhesives with wattle bark tannin was carried out in South Africa [
53
]. Mimosa tannin
adhesives were used instead of synthetic phenolic adhesives to manufacture particleboard and
plywood for external and marine applications [
51
]. In Kenya, the commercial wattle (Acacia mearnsii) is
a well-known tannin-rich species and tannin-based adhesive [
54
]. Current industrialized technologies
are based mostly on paraformaldehyde or hexamethylene tetraamine, which are considered more
environmentally friendly [
55
]. The drive to create more environmentally friendly adhesives has led to
different forms of research in the field; for example, the creation of corn-starch-tannin adhesives in a
study by [56] in a bid to replace synthetic resins has shown that it has excellent structural stability.
8. Nutritive and Antinutritive Effects of Tannins
Tannins, commonly found in most cereal grains and legume seeds, as already indicated,
are considered antinutritional factors that hamper the use of some feeds by monogastric animals. It has
been reported that tannins bind protein, and as a result weakens protein digestion [
57
]. Tannins are
blamed for the bitter taste of the feed, resulting in lowering feed consumption due to reduced
palatability [
58
]. They are regarded as polyphenolic secondary metabolite; however, some reports
have shown recently that low concentrations of some tannin sources can improve the nutrition and
health status of monogastric animals [
2
]. Antinutrients are commonly known as natural or synthetic
compounds that interfere with the absorption of nutrients. Condensed tannins are known to inhibit
several digestive enzymes, including amylases, cellulases, pectinases, lipases, and proteases [
59
].
They have a major antinutritive effect that can influence the nutrient digestibility of lipids, starch,
and amino acids negatively [
60
,
61
]. Tannins are a heterogeneous group of phenolic compounds, found
in nature in many different families of plants. In Oakwood, Trillo, Myrobalaen and Divi-Divi they
occur in almost every part of the plant, such as the leaves, fruits, seed, bark, wood and roots.
Supplementation of chestnut HT at the concentration of 0.5% and 1.0% on rabbit feed had no
effect on growth performance [
62
]. However, [
63
] found different results when chestnut HT was
included in rabbit feed at levels of 0.45% and 0.5%, as it increased feed intake and the live weight
of rabbits. Similarly, [
64
] reported that adding 0.20% of chestnut, the tannin increased average daily
gain and daily feed intake of broilers. The authors of [
65
] reported that when the sweet chestnut
wood extract was used as a supplement at 0.07% and 0.02% for broiler chickens, no antinutritive
Molecules 2020, 25, 4680 6 of 17
activity was observed, and the crude ash, crude protein, calcium and phosphorus were not affected.
The addition of tannic acid (HT) at a dietary level of 0.0125% and 0.1%, showed a negative impact on
hematological indices and plasma iron of pigs [
66
]. According to [
67
], ideal digestibility of energy,
protein, arginine and leucine were lowered in broiler chickens as dietary tannin levels rose to 20 g/kg
diet and beyond, while phenylalanine and methionine were affected negatively only at tannin levels
of 25 g/kg diet. In another study with broiler chickens [
68
], it was reported that the tannin content
of 16 g/kg in red sorghum had no effect on phosphorus, calcium, and nitrogen retention in chickens.
High-tannin sorghum treated with wood ash extract improves its nutritive value [
69
]. Tannins can act
as a double-edged sword; therefore, a tannin content-specific solution could have an effect on their
utilization. Although tanninferous feed and forages containing >5% tannin dry matter are not safe
to be used as animal feed, low to moderate (<5% dry matter) is safe for animal consumption [
59
].
Table 3 shows the antinutritive and nutritive effects of tannins from different plant sources.
Table 3. Nutritive and antinutritive effects of tannins in monogastric animals.
Plant
Source/Tannin
Animal
(Monogastric)
Concentration/Application Effects References
Chestnut (Castanea)
HT
Swine/pig 1%, 2% and 3%
Liver not affected. Changes in
the intestine: villus height
increased, mucosal thickness
and villus perimeter; reduced
large intestinal apoptosis
and mitosis
[70]
Sweet chestnut
wood extract
Chickens
(broilers)
0.07% and 0.2% No antinutritive effects [65]
Tannic acid (TA)
Chickens
(broilers)
1% Tannic acid different
climatic conditions
Better quality of fatty acid
profile of breast muscle
of broilers
[71]
Chestnut (Castanea)
HT
Chickens
(layers)
0.20%
Increased monounsaturated
fatty acid and reduced
cholesterol content of eggs
[72]
Chestnut tannin
extract (Castanea
sativa Miller) HT
Chickens
(layers)
2 g/kg
Unsaturated fatty acids
increased; cholesterol
significantly decreased: −17%
in WLT and −9% in MUT
[73]
High-tannin red
sorghum (Sorghum
vulgaris) HTS
Chickens
(broilers)
16 g/kg (reconstituted red
sorghum)
Utilisations of phosphorus,
nitrogen and calcium retention
were similar
[68]
Chestnut (Castanea)
Pigs 0%, 5%, 10% and 15%
Reduction in digestibility of dry
matter, crude protein, ether
extract, crude ash and tannin
decreased linearly (p < 0.05)
with increasing chestnut
meal supplementation
[74]
9. Influence of Tannins on the Productivity of Monogastric Animals
Tannins have been classified as an “antinutritional factor” for monogastric animals with negative
effects on feed intake, nutrient digestibility, and productionperformance [
1
]. Currently, most researchers
have revealed that some tannins can improve the intestinal microbial ecosystem, enhance gut health,
and hence increase productive performance when applied appropriately in monogastric diets [
62
,
70
,
75
].
Strong protein affinity is a well-recognized property of plant tannins, which has successfully been
applied to monogastric animals’ nutrition. However, adverse effects of high-tannin diets on monogastric
animals’ performance have been reported by many researchers [
71
]. In monogastric animals, the main
effects of tannins are relatedto their protein-bindingcapacityand reduction in protein, starch, and energy
digestibility [
76
,
77
]. According to [
10
,
78
], dry matter intake, bodyweight, feed efficiency and nutrient
digestibility were reduced when chickens were fed diets with tannins, whilst Ebrahim et al. [
71
]

Molecules 2020, 25, 4680 7 of 17
reported a decrease in body weight gain and feed intake. However, [
72
,
75
] reported no effects on growth
performance and on egg weight, cell thickness or yolk color of layers. Several studies showed that low
concentrations of tannins improved feed intake, health status, nutrition, and animal performance in
monogastric farm animals [2,4,79].
According to [
80
], supplementing of pigs’ diet with 0.2% chestnut wood extract rich in tannins
had no effect on growth rate, carcass traits or meat quality of pigs raised up to 26 weeks of age;
whereas Bee et al. [
81
] reported that pigs that were fed diets rich in 3% of hydrolysable tannins from
chestnuts showed no negative effects in terms of growing performance raised from day 105 until
165. The authors of [
49
] reported an increase in small intestinal villus height, villus perimeter and
mucosal thickness in pigs that were fed diets having 3% of hydrolysable tannins from chestnuts.
Moreover, [
4
] reported increased growth performance in pigs aged 23–127 days when fed chestnuts rich
in tannins at the 0.91% supplementation level. According to [
82
], pigs have parotid gland hypertrophy
and secrete proline-rich proteins in the saliva that bind and neutralize the toxic effects of tannins,
which make them relatively resistant to tanniniferous diets without showing any negative effects as
compared to other monogastric animals (Table 3).
In rabbits [
62
], no difference was observed in the performances of rabbits fed diets supplemented
with up to 10 g of tannins from chestnuts. Moreover, they reported that no improvements were observed
in health status, diet nutritive value, growth performance, carcass traits and oxidative stability of rabbits
fed up to 400 g/100 kg of hydrolysable tannins originating from chestnuts. According to [
83
], rabbits fed
diets with 4% of tanniniferous browsers of Acacia karroo, Acacia nilotica and Acacia tortilis showed
no significant differences in intake and digestibility. Mancini et al. [
84
] also reported no significant
difference in growth rate, feed intake or feed conversion ratio and carcass traits of rabbits fed a mixture
of quebracho and chestnut tannins. Moreover, [
85
] observed no significant difference in growth
rate, feed intake or feed conversion ratio of rabbits fed low-tannin sorghum grains. Thus, tannins,
when included in monogastric animal diets, can have both positive and negative effects on animal
performance, depending on the concentration. Therefore, it is important to minimize the inclusion
or supplementation of feedstuffs containing high concentrations of tannins in monogastric animals,
or to take measures to decrease their concentrations. In Table 4, the effect of tannins on productivity of
monogastric animals is reported.
Table 4. Effects of tannins on productivity of monogastric animals.
Tannin
Concentrations
Tannin Source
Monogastric
Animal
Influenced/Affected
Parameter
References
0.16–0.19% Chestnut Pigs
Increased growth
performance
[4]
0.71–1.5% Chestnut Pigs
No effect on feed intake,
body weight gain and
carcass traits; reduced
feed efficiency
[81]
1–3% Chestnut Pigs
Increased small intestinal
villus height, villus
perimeter and
mucosal thickness
[70]
5–10% Grape pomace Broilers
No effect on growth
performance; increased
oxidative stability and
polyunsaturated fatty acids
content of thigh meat
[75]
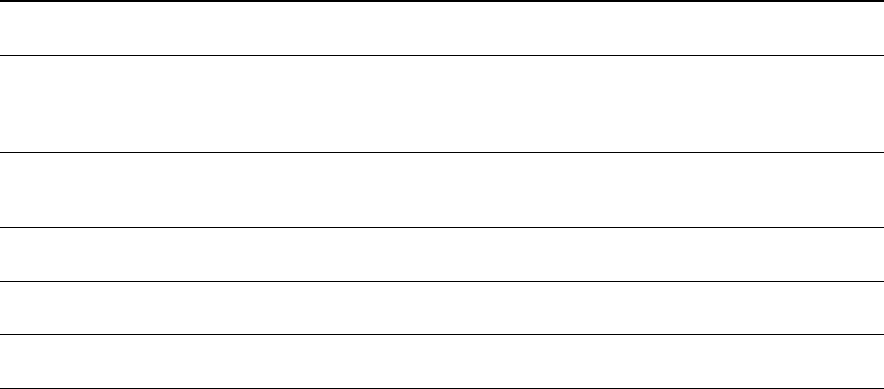
Molecules 2020, 25, 4680 8 of 17
Table 4. Cont.
Tannin
Concentrations
Tannin Source
Monogastric
Animal
Influenced/Affected
Parameter
References
1% Tannic acid Broilers
Decreased body weight gain
and feed intake; improved
the fatty acid profile of
breast muscle
[71]
Chestnut layers
No effect on egg weights,
cell thickness or yolk colour;
reduced cholesterol content
[72]
0.45% and 0.5% Chestnut Rabbits
Increased live weight gain
and feed intake of rabbits
[79,86]
0.5% and 1.0% Quebracho and chestnut Rabbits
Had no effect on growth
performance
[62,84]
4%
Acacia karroo, Acacia
nilotica and Acacia tortilis
Rabbits
No significant differences in
intake and digestibility
[83]
10. Processing Techniques Used to Reduce Effects of Tannins
Several processing techniques to reduce tannin levels in different feedstuffs, especially
unconventional ingredients, have been suggested by most researchers [
86
,
87
]. Processing is an
act of applying suitable techniques to reduce or eliminate tannins present in alternative feedstuffs.
These techniques include enzyme supplementation, soaking, dehulling, alkali treatment, extrusion,
and germination.
10.1. Enzyme Supplementation
Supplementation of enzymes to reduce the tannins content is an effective method, although it
might not be the most economical. It is proven to reduce tannins better than other processing methods,
such as soaking, dehulling, etc. Several studies have shown that enzyme supplementation has been
effective in reducing tannins in alternative energy and protein feedstuffs [
88
,
89
]. A study by [
88
]
found that treatment of sorghum with both polyphenoloxidase and phytase enzymes showed a decrease
in hydrolysable and condensed tannins of 72.3% and 81.3% respectively. Moreover, [
89
] reported a
decrease in both hydrolysable and condensed tannins by 40.6%, 38.92% and 58.00% respectively when
sorghum grains were treated with the three enzymes tannase, phytase and paecilomyces variotii.
10.2. Soaking
Soaking is one of the cheapest traditional methods which animal nutritionists have used for
many years. A study found that the addition of sodium bicarbonate, prolonged time of soaking,
or higher temperature have proved to be effective during the soaking process [
90
]. Kyarisiima et al. [
69
]
reported that high-tannin sorghum soaked in wood ash extract showed a decreased level of tannins
without lowering the nutrient content of sorghum grains. Authors stated that tannin level did not only
decrease with the soaking technique, but also with roasting. The decrease in tannins during soaking
may result from leaching into the soaking water [
77
]. Moreover, [
91
] reported a decrease of about
73–82% in velvet beans.
10.3. Dehulling
Dehulling is a process of reming the outer coat/hull of a seed [
92
]. Most seeds of alternative
feedstuffs have seed coats/hulls which are normally concentrated with tannins. If tannins are removed,
feedstuffs have shown to have a significant increase in protein digestibility and protein content
in legume seed meal. The authors of [
93
] reported that dehulling reduced tannins in chickpea
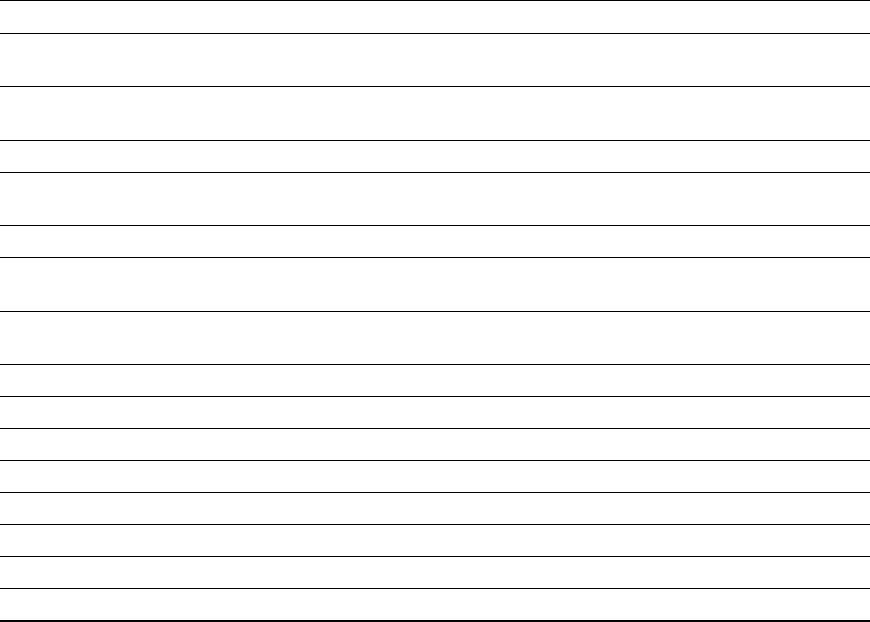
Molecules 2020, 25, 4680 9 of 17
without lowering protein digestibility, whereas in faba beans a 92% decrease of tannins occurred with
dehulling [94].
10.4. Extrusion
The extrusion method is used to decrease levels of tannins in feedstuffs. According to [
95
],
extrusion cooking is a high-temperature, quick process in which starchy food materials are plasticized
and cooked by a combination of moisture, pressure, temperature, and mechanical shear. Extrusion has
shown the ability to inactivate antinutritional elements [
96
–
98
]. For example, [
99
] reportedthatextrusion
showed a significant reduction in tannins with minimum oil loss in flaxseed meal. The authors of [
100
]
reported that lentil splits showed a reduction in tannins after treatment by using extrusion techniques.
Moreover, [101] reported reduction to the extent of 34.52% to 57.41% in sorghum.
10.5. Germination
During the germination process, complex sugars are converted into simple sugars [
91
].
Tannin content has been shown to be reduced by the germination process, which is one of the
cheapest methods. A maximum reduction in tannins of up to 75% has been observed when pearl
millets were treated by using the germination method [
102
]. Rusydi and Azlan [
103
] observed a
reduction of 57.12% when peanuts were treated by using germination. The reduction of tannins may
improve the nutritional quality of feedstuffs. Thus, processing techniques may help to remove or
reduce tannin levels in different feedstuffs, which might be favorable for animal production (Table 5).
Table 5.
Different processing techniques used to reduce the effects of tannins in alternative feedstuffs.
Processing Technique Feedstuff Effectiveness References
Enzyme
supplementation
Sorghum
The enzyme tannase reduced both hydrolysable
and condensed tannins by 40.6%
[89,90]
Dehulling Chickpeas
Reducing tannin level without lowering the
nutrient content of the grain
[94]
Faba beans Reduced about 92% of tannins [95]
Soaking Sorghum
Reducing tannin level without lowering the
nutrient content of the grain
[69,78]
Velvet beans Decreased about 73–82% of tannins [92]
Alkali treatment Sorghum
Reducing tannin level without lowering the
nutrient content of the grain
[78]
Extrusion Flaxseed
Significant reduction of tannins with minimum
oil loss in flaxseed meal
[99]
Lentils Reduced the tannin content in lentil splits [100]
Sorghum Reduction to the extent of 34.52% to 57.41% [101]
Germination Pearl millets Maximum reductions in tannins up to 75% [102]
Peanuts Reduction of tannins by 57.12% [103]
Cooking Cocoyam Reduction of antinutrients in tuber crops [104]
Autoclaving Sorghum Reduction to the extent of 34.52% to 57.41% [101]
Germination Pearl millets Maximum reductions in tannins up to 75% [102]
Peanuts Reduction of tannins by 57.12% [103]
10.6. Cooking
Cooking is considered important in reducing antinutrients activities in tannins. As stated by [
104
],
cooking reduces the antinutrients present in tuber crops like cocoyam.
Molecules 2020, 25, 4680 10 of 17
10.7. Auticlaving
Autoclaving is found to be one of the most effective methods in the elimination of antinutrients,
although it might not be cost effective because of its reliance on electricity [105].
10.8. Grinding
Grinding is considered an effective method in reducing the tannin content because it increases
the surface area which in turn reduces the contact between tannins and the phenolic oxidase in the
plant [106,107].
11. Health Benefits of Tannins in Monogastric Animal Production
Tannins are plant extracts that can be used as additives in monogastric animal feed to control
diseases [
1
].
In vitro
studies have shown that most tannins have antiviral, antibacterial and antitumor
properties [
15
]. Tannins have shown a favorable outcome in the preferment of gut health when used
with other antimicrobials as growth-promoting factors (AGP) such as probiotics [
1
]. Condensed tannins
extracted from green tea or quebracho have shown to have some antimicrobial substances [
108
].
However, [
109
] reported that condensed tannins may have less effect than hydrolysable tannins in
controlling Campylobacter jejuni in the presence of high concentration of amino acids. Moreover, tannins
derived from chestnuts (Castanea sativa) can inhibit the
in vitro
growth of Salmonella typhimurium [
110
].
Several
in vitro
studies have revealed that polyphenols of the procyanidins (CT) have an antioxidant
property while tannic acid has anti-enzymatic, antibacterial and astringent properties, as well as
constringing action on mucous tissues [
111
]. The ingestion of tannic acid causes constipation, so it
can be used to treat diarrhea in the absence of inflammation [
112
]. Kumar et al. [
69
] reported that
the tannin content of 16 g/kg in red sorghum had no effect on certain animal welfare parameters of
broiler chickens. Similarly, globulin, protein, plasma albumin, phosphorus, glucose, calcium, and uric
acid levels were not affected, even when maize is replaced 100% with red sorghum. However, mild
histopathological changes in kidney and liver tissues, as well as high cell-mediated immune response,
were detected when raw red sorghum containing 23 g tannins/kg was fed to the same group of broiler
chickens. The supplementation of purple loosestrife (Lythrum salicaria) in rabbits has led to a significant
increase in the total white blood cells and higher concentrations of volatile fatty acids and acetic acid,
therefore a low level of loosestrife supplementation (<0.4%) has been suggested to gain health benefits
and prevent adverse effects on animal health and performance [113].
Farmatan tannin concentrations of 0.05%, 0.025% and 0.0125% can inhibit the growth of Clostridium
perfringens by more than 54-fold [
114
]. Another
in vitro
study was conducted by [
108
] to evaluate the
effects of tannins from chestnuts and quebracho, or a combination of both, on Clostridium perfringens.
All three products reduced the presence of C. perfringens. When the comparative analysis was
conducted, it was discovered that the concentrations of quebracho tannin were more effective in
inhibiting the growth of C. perfringens as compared to chestnut tannin. Commensal bacteria such as
Bifidobacterium breve or Lactobacillus salivarius are very useful and their growth or presence should not be
inhibited by the tannin. Kamijo et al. [
115
] reported that ellagitannins isolated from Rosa rugose petals
have some antibacterial activities against pathogenic bacteria such as Salmonella sp, Bacillus cereus,
S. aureus and E. coli but they had no effect on beneficial bacteria. Most
in vitro
results are supported
by
in vivo
experiments that the inclusion of tannin in monogastric animals can lower the occurrence
and severity of diarrhea [
116
]. However, the efficiency of adding tannins that shows robustness
in inhibiting pathogens in
in vitro
studies needs to be evaluated further in the experimental set-up
(
in vivo
) involving poultry and pigs. These disparities in terms of types of tannins that are efficient
in combating certain pathogens warrant further research. Table 6 shows different health benefits of
tannins in monogastric animals.
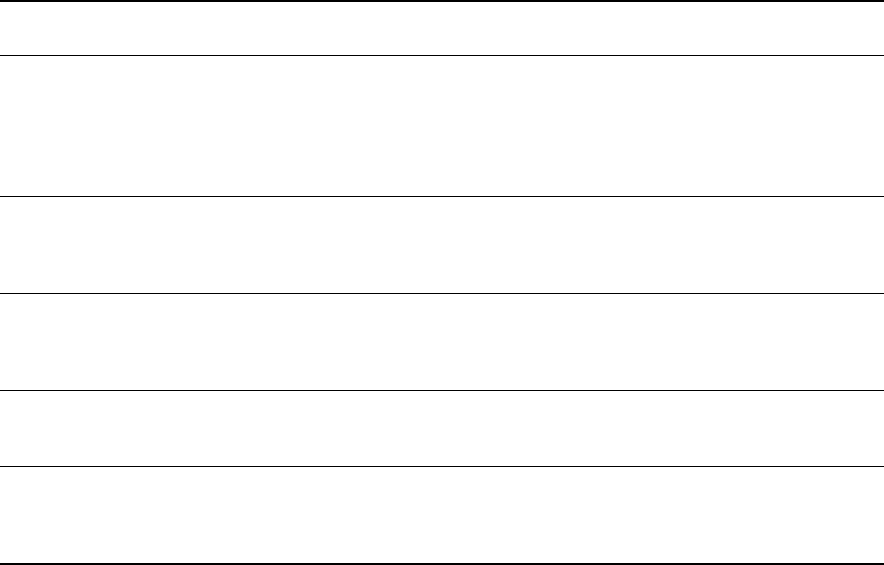
Molecules 2020, 25, 4680 11 of 17
Table 6. Health benefits of tannins in monogastric animals.
Plant
Source/Tannin
Animal/Monogastric
Application
Rates
Health Benefits References
Chestnut
tannin (HT)
Chickens
0, 250, 500 and
1000 mg/kg
250 mg/kg reduced number of
E. coli and coliform bacteria in
small intestine. Greatest
number of Lactobacillus
observed in supplementation
of 1000 mg/kg
[49]
Purple
loosestrife
(Lythrum
salicatia)
Rabbit
0.2%, 0.4% and
0.3%
Increased total white blood
cells in rabbit
[113]
Chestnut (HT) Chickens (broiler) 0.15% to 1.2%
Reduced bacteria in the gut.
Clostridium perfringens
(Eimeria maxima, Eimeria tenella
and Eimeria acervulina)
[117]
Grape pomace
(CT)
Pigs 2.80%
Reduction in the absorption of
mycotoxins in the
gastrointestinal surface
[118]
Grape pomace
(CT)
Chickens (broiler) 6%
Increased commensal bacteria
(Lactobacillus) and decreased
the counts of clostridium
bacteria in ileal content
[118]
12. Conclusions
In the quest to find alternative feed ingredients in the production of monogastric animals, the effects
of tannins have proven to be of value. Tannins can be beneficial in both as feed ingredients and a valuable
ingredient in animal health. Although tannins contain antinutrients, different processing methods have
proved to be effective in the reduction or elimination of these antinutrients. This review has provided
extensive literature on the benefits and impacts of tannins in poultry production. Furthermore, it has
elaborated on the different processing methods which can be employed to reduce the negative effects
of tannins. The methods chosen should be cost-effective, easy to use and should not defeat the purpose
of alternative feed ingredients. Even though tannins can act as feed additives, their inclusion level
will depend on the source, age and species of poultry. Thus, future research should focus on the
optimum tannin inclusion level in poultry and more cost-effective processing methods, especially for
small-scale poultry keepers who mostly utilize these alternative feed ingredients. The development of
more convenient readily available products of tannins ready to be incorporated in the monogastric
animal feed is encouraged.
Author Contributions:
Conceptualization, M.M.; Writing—original draft preparation, T.G.M., L.S. and Z.M.H.;
Review and editing M.M.; Visualization M.M. All authors have read and agreed to the published version of
the manuscript.
Funding: The authors would like to thank the University of South Africa for the financial support.
Conflicts of Interest: The authors declare no conflict of interest.
References
1.
Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez, M.E. Perspectives in the use of tannins as
alternative to antimicrobial growth promoter factors in poultry. Front Microbiol. 2014, 5, 118. [CrossRef]
2.
Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed
antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [CrossRef]
3. Butler, L.G. Antinutritional effects of condensed and hydrolyzable tannins. Basic Life Sci. 1992, 59, 693–698.
Molecules 2020, 25, 4680 12 of 17
4.
Brus, M.; Dolin, J.; CenCi, C.A.; Skorjanc, D. Effect of chestnut (Castanea sativa) wood tannins and organic
acids on growth performance and faecal microbiota of pigs from 23 to 127 days of age. Bulg. J Agric. Sci.
2013, 19, 841–847.
5.
Biagia, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect of tannins on growth performance and intestinal
ecosystem in weaned piglets. Arch. Anim. Nutr. 2010, 64, 121. [CrossRef]
6.
Wang, M.L.; Suo, X.; Gu, J.H.; Zhang, W.W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin
extract in broiler chickens: Effect on chicken coccidiosis and antioxidant status. Poult. Sci.
2008
, 87, 2273–2280.
[CrossRef]
7.
Maunder, B. Sorghum—The Global Grain of the Future. Available online: http://www.sorghumgrowers.com/
maunder.html (accessed on 26 August 2020).
8.
Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.;
Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement.
PLoS Med. 2009, 6, e1000097. [CrossRef]
9.
Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev.
1988
, 1, 209–231. [CrossRef]
[PubMed]
10.
Hassan, I.A.; Elzuber, E.A.; Tinay, H.A. Growth and apparent absorption of minerals in broiler chicks fed
diets with low or high tannin content. Trop. Anim. Hlth. Prod. 2003, 35, 189–196. [CrossRef] [PubMed]
11.
Hagerman, A.E.; Butler, L.G. Tannins and lignins. In Herbivores: Their Interactions with Secondary Plant
Metabolites; Rosenthal, G.A., Berbenbaum, M.R., Eds.; Academic Press: New York, NY, USA, 1991; pp. 355–388.
12.
Barbehenn, R.V.; Constable, C.P. Tannins in plant-herbivore interactions. Phytochemistry
2011
, 72, 1551–1565.
[CrossRef] [PubMed]
13. Woller, R.; Würdig, G. Chemie des Weines; Ulmer: Stuttgart, Germany, 1989.
14.
Porter, L.J. Methods in plant biochemistry. In Plant Phenolics; Harborne, J.B., Ed.; Academic Press: London,
UK, 1989.
15. Khanbabaee, K.; Van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 1, 641–649.
16.
Fraga-Corral, M.; Garc
í
a-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.;
Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules
2020
, 25, 614. [CrossRef]
[PubMed]
17.
Brillouet, J.M.; Romieu, C.; Schoefs, B. The tannosome is an organelle forming condensed tannins in the
chlorophyllous organs of Tracheophyta. Ann. Bot. 2013, 112, 1003–1014. [CrossRef] [PubMed]
18.
Arbenz, A.; Av
é
rous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular
architectures. Green Chem. 2015, 17, 2626–2646. [CrossRef]
19.
Grasel, F.S.; Ferrao, M.F. A rapid and non-invasive method for the classification of natural tannin extracts by
nearinfrared spectroscopy and PLS-DA. Anal. Methods 2016, 8, 644–649. [CrossRef]
20.
Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric proanthocyanidins. Stereochemistry,
structural units, and molecular weight. J. Chem. Soc. Perkin Trans. 1980, 1, 2278–2286. [CrossRef]
21. Hagerman, A.E. Tannin-protein interactions. ACS Symp. Ser. 1992, 506, 236–247.
22.
Isam, E.; Hussein, E.; Ishak, C.Y. Determination of tannins of three common Acacia species of Sudan.
Adv. Chem. 2014. [CrossRef]
23.
Amarowicz, R. Tannins: The new natural antioxidants? Eur. J. Lipid Sci. Technol.
2007
, 109, 549–551.
[CrossRef]
24.
Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L. Condensed and hydrolysable tannins
as antioxidants influencing the health. Min. Rev. Med. Chem. 2008, 8, 436–447. [CrossRef]
25.
Galloway, D.F. The Biological Significance of Tannins: An Overview. In Chemistry and Significance of Condensed
Tannins; Hemingway, R.W., Karchesy, J.J., Branham, S.J., Eds.; Springer: Boston, MA, USA, 1989.
26.
Sing, A.P.; Kumar, S. Applications of Tannins in Industry. Structural properties, Biological properties and
Current knowledge. IntechOpen 2019. [CrossRef]
27.
Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.;
Fouuld, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions.
Lancet Infect Dis. 2013
,
13, 1057–1098. [CrossRef]
28.
Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig.
2003
, 111,
1265–1273. [CrossRef] [PubMed]
Molecules 2020, 25, 4680 13 of 17
29.
White, D.G.; Zhao, S.; Sudler, R.; Ayers, S.; Friedman, S.; Chen, S.; McDermott, P.F.; McDermott, S.;
Wagner, D.D.; Meng, J. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl.
J. Med. 2011, 345, 1147–1154. [CrossRef] [PubMed]
30.
Angulo, F.J.; Nunnery, J.A.; Bair, H.D. Antimicrobial resistance in zoonotic enteric pathogens. Rev. Sci. Tech.
2004, 23, 485–496. [CrossRef]
31.
Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to
human health: How worried should we be? Evol. Appl. 2015, 8, 240–245. [CrossRef]
32.
Porrero, M.C.; Mentaberre, G.; S
á
nchez, S.; Fern
á
ndez-Llario, P.; Casas-D
í
az, E.; Mateos, A.; Vidal, D.;
Lav
í
n, S.; Fern
á
ndez-Garayz
á
bal, J.-F.; Dom
í
nguez, L. Carriage of Staphylococcus aureus by free-living wild
animals in Spain. Appl. Environ. Microbiol. 2014, 80, 4865–4870. [CrossRef]
33.
Alali, W.Q.; Thakur, S.; Berghaus, R.D.; Martin, M.P.; Gebreyes, W.A. Prevalence and distribution of
Salmonella in organic and conventional poultry farms. Foodborne Pathog. Dis.
2010
, 7, 1363–1371. [CrossRef]
34.
Kim, J.M.; Hong, J.; Bae, W.; Koo, H.C.; Kim, S.H.; Park, Y.H. Prevalence, antibiograms, and transferable
tet(O)plasmid of Campylobacter jejuni and Campylobacter coli isolated from raw chicken, pork, and human
clinical cases in Korea. J. Food Prot. 2010, 73, 1430–1437. [CrossRef]
35.
Korotkevich Yu, V. Antibiotic resistance analysis of Enterococcus spp. and Enterobacteriaceae spp. isolated
from food. Probl. Nutr. 2016, 85, 5–13.
36.
Altalhi, A.D.; Gherbawy, Y.A.; Hassan, S.A. Antibiotic Resistance in Escherichia coli Isolated from Retail Raw
Chicken Meat in Taif, Saudi Arabia. Foodborne Pathog. Dis. 2009. [CrossRef]
37.
Sheikh, A.A.; Checkley, S.; Avery, B.; Chalmers, G.; Bohaychuk, V.; Boerlin, P.; Reid-Smith, R.; Aslam, M.
Antimicrobial Resistance and Resistance Genes in Escherichia coli Isolated from Retail Meat Purchased in
Alberta, Canada. Foodborne Pathog. Dis. 2012, 9. [CrossRef] [PubMed]
38.
Gedir, J.V.; Sporns, P.; Hudson, R.J. Extraction of condensed tannins from cervid feed and faeces and
quantification using a radial diffusion assay. J. Chem. Ecol. 2005, 31, 2761–2773. [CrossRef]
39.
Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Duran, N. Phenolic compounds
and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [CrossRef]
40.
Haslam, E. Natural polyphenols (Vegetatable Tannins) as drugs: Possible modes of action. J. Nat. Prod.
1996
,
59, 205–215. [CrossRef] [PubMed]
41.
Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food
Sci. Nutr. 1998, 38, 421–464. [CrossRef] [PubMed]
42.
Sieniawska, E.; Baj, T. Tannins. In Pharmacognosy. Fundamentals, Applications and Strategies; Simone, B.-M.,
Rupika, D., Eds.; Academic Press: Cambridge, UK, 2017.
43.
Graziani, R.; Tosi, G.; Denti, R.
In vitro
antimicrobial activity of Silvafeed ENC on bacterial strains of poultry
origin. In Proceedings of the 12th European Poultry Conference, Verona, Italy, 10–14 September 2006.
44. Li, Y.; Song, G. Study on bacteriostasis of chestnut shell extract. Chem. Ind. For. Prod. 2004, 4, 61–64.
45.
Özcan, M. Effect of sumach (Rhus coriaria L.) extracts on the oxidative stability of peanut oil. J. Med. Food
2003, 86, 2010–2037. [CrossRef]
46.
Kaur, K.; Husheem, M.; Arora, S.; Härkönen, P.; Subodh, K.K.
In vitro
bioactivity-guided fractionation and
characterization of polyphenolic inhibitory fractions from Acacia nilotica (L.). J. Ethnopharmacol
2005
, 99,
353–360. [CrossRef]
47.
Costabile, A.; Sanghi, S.; Mart
í
n-Pelaez, S.; Mueller-Harvey, I.; Gibson, G.R.; Rastall, R.A.; Klinder, A.
Inhibition of Salmonella Typhimurium by tannins in vitro. J. Food Agric. Environ. 2011, 9, 119–124.
48.
Attia, M.F.A.; El-din, A.N.M.N.; El-zarkouny, S.Z.; El-zaiat, H.M. Impact of Quebracho Tannins
Supplementation on Productive and Reproductive Efficiency of Dairy Cows. Sci. J. Anim. Sci.
2016
,
6, 269–288. [CrossRef]
49.
Tosi, G.; Massi, P.; Antongiovanni, M.; Buccioni, A.; Minieri, S.; Marenchino, L.; Mele, M. Efficacy Test of a
Hydrolysable Tannin Extract Against Necrotic Enteritis in Challenged Broiler Chickens. Ital. J. Anim. Sci.
2013, 12, e62. [CrossRef]
50.
Hur, S.N.; Molan, A.L.; Cha, J.O. Effects of Feeding Condensed Tannin-containing Plants on Natural Coccidian
Infection in Goats. Asian-Aust. J. Anim. Sci. 2005, 18, 1262–1266.
51.
Zhou, X.; Du, G. Applications of tannin resin adhesives in the wood industry. In Tannins—Structural Properties,
Biological Properties, and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2019.
Molecules 2020, 25, 4680 14 of 17
52.
Custers, P.A.J.L.; Rushbrook, R.; Pizzi, A.; Knauff, C.J. Industrial applications of
wattle-tannin/urea-formaldehyde fortified starch adhesives for damp-proof corrugated cardboard.
Holzverwert. 1979, 31, 131–133.
53.
Saayman, H.M.; Brown, C.H. Wattle-base tannin-starch adhesives for corrugated containers. Forest Prod. J.
1977, 27, 21–25.
54.
Mugedo, J.Z.A.; Waterman, P.G. Sources of tannin: Alternatives to wattle (Acacia mearnsii) among indigenous
Kenyan species. Econ. Bot. 1992, 46, 55–63. [CrossRef]
55.
Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules
2019
, 9, 344. [CrossRef]
[PubMed]
56.
Moubarik, A.; Ahmed, A.; Pizzi, A.; Charrier, F. Preparation and mechanical characterization of particle
board made from maritime pine and glued with bio-adhesives based on cornstarch and tannins.
Maderas. Cienc. Tecnol. 2010, 12, 189–197. [CrossRef]
57.
Medugu, C.I.; Saleh, B.; Igwebuike, J.U.; Ndirmbita, R.L. Strategies to improve the utilization of tannin-rich
feed materials by poultry. Int. J. Poult. Sci. 2012, 11, 417.
58.
Butler, L.G.; Riedl, D.J.; Lebryk, D.G.; Blytt, H.J. Interaction of proteins with sorghum tannin: Mechanism,
specificity and significance. J. Am. Oil Chem. Soc. 1984, 61, 916–920. [CrossRef]
59.
Bhat, T.K.; Kannan, A.; Singh, B.; Sharma, O.P. Value addition of feed and fodder by alleviating the
antinutritional effects of tannins. Agric. Res. 2013, 2, 189–206. [CrossRef]
60.
Garcia, R.G.; Mendes, A.A.; Sartori, J.R.; de Lima Almeida Paz, I.C.; Takahashi, S.E.; Pel
í
cia, K.;
Komiyama, C.M.; Quinteiro, R.R. Digestibility of feeds containing sorghum, with and without tannin,
for broiler chickens submitted to three room temperatures. Braz. J. Poult. Sci. 2004, 6, 55–60. [CrossRef]
61.
Brestensk
ý
, M.; Nitrayov
á
, S.; Patr
á
s, P.; Heger, J. The quality of sorghum grain in aspect of utilization amino
acids in pigs. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 1032.
62.
Liu, H.W.; Zhou, D.; Tong, J.; Vaddella, V. Influence of chestnut tannins on welfare, carcass characteristics,
meat quality, and lipid oxidation in rabbits under high ambient temperature. Meat Sci.
2012
, 90, 164–169.
[CrossRef] [PubMed]
63.
Zoccarato, I.; Gasco, L.; Schiavone, A.; Guo, K.; Barge, P.; Rotolo, L.; Savarino, G.; Masoero, G. Effect of extract
of chestnut wood inclusion (ENC
®
) in normal and low protein ammino acid supplemented diets on heavy
broiler rabbits. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; pp. 873–878.
64.
Schiavone, A.; Guo, K.; Tassone, S.; Gasco, L.; Hernandez, E.; Denti, R.; Zoccarato, I. Effects of a natural
extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult. Sci.
2008, 87, 521–527. [CrossRef]
65.
Rezar, V.; Salobir, J. Effects of tannin-rich sweet chestnut (Castanea sativa) wood extract supplementation on
nutrient utilisation and excreta dry matter content in broiler chickens. Eur. Poult. Sci. 2014, 78, 1–10.
66.
Lee, S.H.; Shinde, P.L.; Choi, J.Y.; Kwon, I.K.; Lee, J.K.; Pak, S.I.; Cho, W.T.; Chae, B.J. Effects of tannic acid
supplementation on growth performance, blood haematology, iron status and faecal microflora in weanling
pigs. Livest. Sci. 2010, 131, 281–286. [CrossRef]
67.
Iji, P.A.; Khumalo, K.; Slippers, S.; Gous, R.M. Intestinal Function and Body Growth of Broiler Chickens on
Maize-based Diets Supplemented with Mimosa Tannins and a Microbial Enzyme. J. Sci. Food Agric.
2004
, 84,
1451–1458. [CrossRef]
68.
Kumar, V.; Elangovan, A.V.; Mandal, A.B.; Tyagi, P.K.; Bhanja, S.K.; Dash, B.B. Effects of feeding raw or
reconstituted high tannin red sorghum on nutrient utilisation and certain welfare parameters of broiler
chickens. Br. Poult. Sci. 2007, 48, 198–204. [CrossRef]
69.
Kyarisiima, C.C.; Okot, M.W.; Svihus, B. Use of wood ash in the treatment of high tannin sorghum for poultry
feeding. S. Afr. J. Anim. Sci. 2004, 34, 110–115. [CrossRef]
70.
Bili´c-Šobot, D.; Kubale, V.; Škrlep, M.;
ˇ
Candek-Potokar, M.; Prevolnik Povše, M.; Fazarinc, G.; Škorjanc, D.
Effect of hydrolysable tannins on intestinal morphology, proliferation and apoptosis in entire male pigs.
Arch. Anim. Nutr. 2016, 70, 378–388. [CrossRef] [PubMed]
71.
Ebrahim, R.; Liang, J.B.; Jahromi, M.F.; Shokryazdan, P.; Ebrahimi, M.; Li, C.W.; Goh, Y.M. Effects of tannic
acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Ital. J.
Anim. Sci. 2015, 14, 3956. [CrossRef]
72.
Antongiovanni, M.; Minieri, S.; Buccioni, A.; Galigani, I. Effect of a tannin from chestnut wood
(Castanea sativa Miller) on cholesterol and fatty acids of eggs. Eur. Symp. Qual. Poult. Meat 2015, 22, 52.
Molecules 2020, 25, 4680 15 of 17
73.
Minieri, S.; Buccioni, A.; Serra, A.; Galigani, I.; Pezzati, A.; Rapaccini, S.; Antongiovanni, M. Nutritional
characteristics and quality of eggs from laying hens fed on a diet supplemented with chestnut tannin extract
(Castanea sativa Miller). Br. Poult. Sci. 2016, 57, 824–832. [CrossRef]
74.
Lee, H.J.; Choi, I.H.; Kim, D.H.; Amanullah, S.M.; Kim, S.C. Nutritional characterization of tannin rich
chestnut (Castanea) and its meal for pigs. J. Appl. Anim. Res. 2016, 44, 258–262. [CrossRef]
75.
Chamorro, S.; Viveros, A.; Rebol
´
e, A.; Rica, B.D.; Arija, I.; Brenes, A. Influence of dietary enzyme addition on
polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Food Res. Int.
2015
, 73, 197–203.
[CrossRef]
76.
Houshmand, M.; Hojati, F.; Parsaie, S. Dietary nutrient manipulation to improve the performance and tibia
characteristics of broilers fed oak acorn (Quercus Brantii Lindl). Braz. J. Poult. Sci.
2015
, 17, 17–24. [CrossRef]
77.
Tapiwa, K.A. Polyphenols in sorghum, their effects on broilers and methods of reducing their effects:
A Review. Biomed. J. Sci. Tech. Res. 2019, 19, 14058–14061. [CrossRef]
78.
Ravindran, G.; Ravindran, V.; Bryden, W.L. Total and ileal digestible tryptophan contents of feedstuffs for
broiler chickens. J. Sci. Food Agric. 2006, 86, 1132–1137. [CrossRef]
79.
Maertens, L.; Štruklec, M. Technical note: Preliminary results with a tannin extract on the performance and
mortality of growing rabbits in an enteropathy infected environment. World Rabbit Sci.
2006
, 14, 189–192.
[CrossRef]
80.
Prevolnik, M.; Skrlep, M.; Brus, M.; Pugliese, C.; Candek-Potokar, M.; Skorjanc, D. Supplementing pig diet
with 0.2% sweet chestnut (Castanea sativa Mill) wood extract had no effect on growth, carcass or meat quality.
Acta Agric. Slov. 2012, 3, 83–88.
81.
Bee, G.; Silacci, P.; Ampuero-Kragten, S.;
ˇ
Candek-Potokar, M.; Wealleans, A.L.; Litten-Brown, J.; Salminen, J.P.;
Mueller-Harvey, I. Hydrolysable tannin-based diet rich in gallotannins has a minimal impact on pig
performance but significantly reduces salivary and bulbourethral gland size. Animal
2016
, 11, 1617–1625.
[CrossRef] [PubMed]
82.
Cappai, M.G.; Wolf, P.; Dimauro, C.; Pinna, W.; Kamphues, J. The bilateral parotidomegaly (hypertrophy)
induced by acorn consumption in pigs is dependent on individual’s age but not on intake duration. Livest Sci.
2014, 167, 263–268. [CrossRef]
83.
Mashamaite, L.; Ng’ambi, J.W.; Norris, D.; Ndlovu, L.R.; Mbajiorgu, C.A. Relationship between Tannin
Contents and Short-Term Biological Responses in Male Rabbits Supplemented with Leaves of Different
Acacia Tree Species Grown in Limpopo Province of South Africa. Livestock Research for Rural Development.
Available online: http://www.lrrd.org/lrrd21/7/mash21109.html (accessed on 26 August 2020).
84.
Mancini, S.; Moruzzo, R.; Minieri, S.; Turchi, B.; Cerri, D.; Gatta, D.; Sagona, S.; Felicioli, A.; Paci, G. Dietary
supplementation of quebracho and chestnut tannins mix in rabbit: Effects on live performances, digestibility,
carcase traits, antioxidant status, faecal microbial load and economic value. Ital. J. Anim. Sci.
2019
, 18,
621–629. [CrossRef]
85.
Al-Mamary, M.; Molham, A.; Abdulwali, A.; Al-Obeide, A.
In vivo
effect of dietary sorghum tannins on
rabbit digestive enzyme and mineral absorption. Nutr. Res. Rev. 2001, 21, 1393–1401. [CrossRef]
86.
Agume, A.S.; Njintang, N.Y.; Mbofung, C.M. Effect of soaking and roasting on the physicochemical and
pasting properties of soybean flour. Foods 2017, 6, 12. [CrossRef]
87.
Vagadia, B.V.; Sai, K.V.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor. A review. Trends Food
Sci. Tech. 2017, 64. [CrossRef]
88.
Avil
´
es-Gaxiola, S.; Chuck-Hernandez, C.; Sergio, O.; Sald
´
ıvar, S. Inactivation Methods of Trypsin Inhibitor
in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [CrossRef]
89.
Towo, E.; Matuschek, E.; Svanberg, U. Fermentation and enzyme treatment of tannin sorghum gruels: Effects
on phenolic compounds, phytate and in vitro accessible iron. Food Chem. 2006, 94, 369–376. [CrossRef]
90.
Schons, P.; Fernanda, B.; Macedo, V.; Alves, G. Fermentation, and enzyme treatments for sorghum.
Braz. J. Microbiol. 2012, 43, 89–97. [CrossRef]
91.
Iji, P.; Toghyani, M.; Ahiwe, E.; Omede, A.A. Alternative Sources of Protein for Poultry Nutrition. 2017.
Available online: http://dx.doi.org/10.19103/AS.2016.0011.13 (accessed on 31 August 2020).
92.
Vadivel, V.; Pugalenthi, M. Removal of anti-nutritional/toxic substances and improvement in the protein
digestibility of velvet bean (Mucuna pruriens) seeds during processing. J. Food Sci. Technol.
2008
, 45, 242–246.
93.
Sunil, C.K.; Chidanand, D.V.; Manoj, D.; Choudhary, P.; Rawson, A. Effect of ultrasound treatment on
dehulling efficiency of blackgram. J. Food Sci. Technol. 2018, 55, 2504–2513. [CrossRef]
Molecules 2020, 25, 4680 16 of 17
94.
Mittal, R.; Nagi, H.P.S.; Sharma, P.; Sharma, S. Effect of Processing on Chemical Composition and
Antinutritional Factors in Chickpea Flour. J. Food Sci. Eng. 2012. [CrossRef]
95.
Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients
and
in vitro
digestibility of protein and starch in faba and kidney beans. Food Chem.
2000
, 68, 159–165.
[CrossRef]
96.
Navale, S.A.; Swami, S.B.; Thakor, N.J. Extrusion Cooking Technology for Foods: A Review. J. Ready Eat Food.
2015, 2, 66–80.
97.
Kaur, S.; Sharma, S.; Singh, B.; Dar, B. Effect of extrusion variables (temperature, moisture) on the antinutrient
components of cereal brans. J. Food Sci. Technol. 2015, 52, 1670–1676. [CrossRef] [PubMed]
98.
Rahul, P.R.; Uday, S.A. Effect of extrusion process on antinutritional factors and protein and starch digestibility
of lentil splits. Food Sci. Technol. 2016, 66, 114–123.
99.
Muhammad, I.; Faqir, A.; Butt, M.; Sheikh, M. Influence of Extrusion Processing on Tannin Reduction and
Oil Loss in Flaxseed (Linum Usitatissimum L.) Meal. J. Food Process. Preserv. 2014, 38. [CrossRef]
100.
Kumar, A.; Mani, I.; Aradwad, P.; Samuel, D.V.K.; Jha, S.; Sahoo, P.K.; Sinha, J.P.; Kar, A. Effect of extrusion
technique on anti-nutritional factors of sorghum-soya blends. Indian J. Agric. Sci. 2018, 88, 81–89.
101.
Singh, A.; Gupta, S.; Kaur, R.; Gupta, H.R. Process optimization for anti-nutrient minimization of millets.
Asian J. Dairy Food Res. 2017, 36, 1–5. [CrossRef]
102.
Rusydi, M.R.; Azlan, A. Effect of germination on total phenolic, tannin and phytic acid contents in soy bean
and peanut. Int. Food Res. J. 2012, 19, 673–677.
103.
Agwunobi, L.N.; Angwukam, P.O.; Cora, O.O.; Isika, M.A. Studies on the use of colocasia. esculenta
(Taro cocoyam) in the diets of weaned pigs. Trop. Anim. Health Prod.
2002
, 34, 241–247. [CrossRef] [PubMed]
104.
Abeke, F.O.; Otu, M. Antinutrients in poultry feeds: Concerns and options. In Proceedings of the 13th Annual
Conferenceof the Animal Science Association of Nigeria (ASAN), A.B.U Zaria, Nigeria,
15–19 September 2008
;
pp. 396–398.
105.
Manach, C.; Scalbert, A.; Morand, C.; Re
´
me
´
sy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability.
Am. J. Clin. Nutr. 2004, 79, 727–747. [CrossRef] [PubMed]
106.
Vitti, D.M.S.S.; Nozella, E.F.; Abdalla, A.L.; Bueno, I.C.S.; Silva Filho, J.C.; Costa, C.; Bueno, M.S.; Longo, C.;
Vieira, M.E.Q.; Cabral Filho, S.L.S.; et al. The effect of drying and urea treatment on nutritional and
anti-nutritional components of browses collected during wet and dry seasons. Anim. Feed Sci. Technol.
2005
,
122, 123–133. [CrossRef]
107.
Elizondo, A.M.; Mercado, E.C.; Rabinovitz, B.C.; Fernandez-Miyakawa, M.E. Effect of tannins on the
in vitro
growth of Clostridium perfringens. Vet. Microbial. 2010, 145, 308–314. [CrossRef]
108.
Anderson, R.C.; Vodovnik, M.; Min, B.R.; Pinchak, W.E.; Krueger, N.A.; Harvey, R.B.; Nisbet, D.J. Bactericidal
effect of hydrolysable and condensed tannin extracts on Campylobacter jejuni
in vitro
. Folia Microbial.
2012
,
57, 253–258. [CrossRef]
109.
Van Parys, A.; Boyen, F.; Dewulf, J.; Haesebrouck, F.; Pasmans, F. The use of tannins to control Salmonella
typhimurium infections in pigs. Zoonoses Public Health 2010, 57, 423–428. [CrossRef]
110.
Corder, R. Tannins in Some European Reds have Greater Protective Effects on Heart than Other Red Wines.
Nature Online Advance Edition. News release, Queen Mary University of London. Available online:
http://www.cbsnews.com/stories/2006/11/29/health/webind/main2215429.html (accessed on 5 August 2020).
111.
Phytolab. Phytochemicals Polyphenols—Tannic Acid. Available online: http://www.
phytochemicalsinfolphytochemicals/tannic-acid.phD. (accessed on 26 July 2020).
112.
Kovitvadhi, A.; Gasco, L.; Ferrocino, I.; Rotolo, L.; Dabbou, S.; Malfatto, V.; Gai, F.; Peiretti, P.G.; Falzone, M.;
Vignolini, C.; et al. Effect of purple loosestrife (Lythrum salicaria) diet supplementation in rabbit nutrition on
performance, digestibility, health and meat quality. Animal 2016, 10, 10–18. [CrossRef]
113.
Bole-Hribovsek, V.M.; Drobnic-Kosorek, J.; Ponebsek, D.; Moran, D.M. Minimum inhibitory concentrations
of Farmatan dried all-natural tannic acid (flavoring) feed additive on pathogenic strains of Clostridium
perfringens and Escherichia coli plus isolates of four salmonella species
in vitro
. In Proceedings of the Poultry
Science Assn. annual meeting, Athens, Greece, 9–12 July 2012.
114.
Kamijo, M.; Kanazawa, T.; Funaki, M.; Nishizawa, M.; Yamagishi, T. Effects of Rosa rugosa petals on intestinal
bacteria. Biosci. Biotechnol. Biochem. 2008, 72, 773–777. [CrossRef]
115.
Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform
diarrhea in weaned pigs. Animal 2020, 14, 95–107. [CrossRef]

Molecules 2020, 25, 4680 17 of 17
116.
Jamroz, D.; Wiliczkiewicz, A.; Skorupi´nska, J.; Orda, J.; Kuryszko, J.; Tschirch, H. Effect of sweet chestnut
tannin (SCT) on the performance, microbial status of intestine and histological characteristics of intestine
wall in chickens. Br. J. Poult. Sci. 2009, 50, 687–699. [CrossRef] [PubMed]
117.
Gambacorta, L.; Pinton, P.; Avantaggiato, G.; Oswald, I.P.; Solfrizzo, M. Grape pomace, an agricultural
byproduct reducing mycotoxin absorption:
In vivo
assessment in pig using urinary biomarkers. J. Agric.
Food Chem. 2016, 64, 6762–6771. [CrossRef] [PubMed]
118.
Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich
grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci.
2011
, 90, 566–578.
[CrossRef]
Publisher’s Note:
MDPI stays neutral with regard to jurisdictional claims in published maps and institutional
affiliations.
©
2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access
article distributed under the terms and conditions of the Creative Commons Attribution
(CC BY) license (http://creativecommons.org/licenses/by/4.0/).